alkaline earth metal
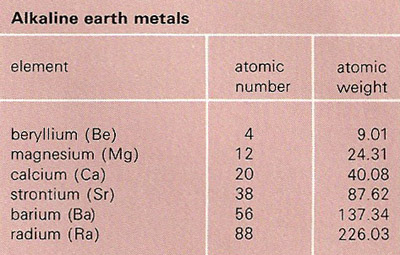
An alkaline earth metal is any of the six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Alkaline earth metals make up group IIA of the periodic table of elements. They all exhibit a single oxidation state, +2, are light, and are reactive, though less so than the alkali metals. Barium and radium are the most reactive and beryllium is the least.
In the past, chemists used the term "earth" to denote slightly soluble metal oxides. The oxides of barium, strontium, and calcium resemble alumina (Al2O3), a typical "earth", but form alkaline mixtures with water. For this reason barium, strontium, and calcium were called alkaline earth metals. This name was later extended to include all of the elements of group 2.


