beta particle
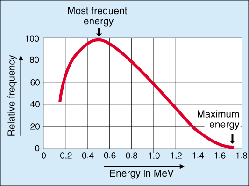
Energy distribution of electrons (beta-particles) emitted during the beta decay of P-32. Credit: European Nuclear Society.
A beta particle is a fast-moving electron or positron (anti-electron) that is emitted from a nucleus during the radioactive process known as beta decay. Large amounts of beta radiation may cause skin burns, and beta emitters are harmful if they enter the body. Beta particles may be stopped by thin sheets of metal or plastic.
Most neutron-rich or neutron-deficient atoms that are lighter than lead decay by beta decay. What happens is that a neutron changes into a proton or vice versa. In the transformation n → p the nucleus loses a negative charge and emits an electron, e-. In the transformation p → n the nucleus loses a positive charge and emits a positron, e+. For example, 14-C → 14-N + e- or 18-F → 18-O + e+.
Beta particles travel with an initial speed of about 180 million m/s, or about 0.6 light-speed. Because they are charged they will interact with electrons in atoms they come close to and cause ionization. A medium energy beta particle will travel about one meter in air but only about one millimeter through body tissue.
Beta radiation has an energy continuum. The maximum energy Eβmax is always quoted, e.g. for P-32 decay this is 1.7 MeV (see graph). Beta rays span an energy range of 0.003–13 MeV, and their distribution is characteristic of the emitting isotope.
Beta particles were discovered by Henri Becquerel in 1876, although at that time their nature was not understood.
See also alpha particle and gamma rays.


