ionization potential
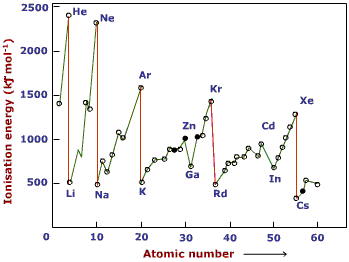
Energy is needed to remove a single electron from an atom of any element. The amount needed, called the first ionization potential, is different for each element. When all these energies are plotted on a graph, with the elements in order of increasing atomic number along the bottom, it can be seen that they vary in a periodic manner, rising gradually and then suddenly falling, before rising again.
The ionization potential is the energy needed to remove an electron from the ground state of a given type of atom to infinity. It increases for removal of successive electrons, which are bound to the atom's positive charge. Ionization potentials can be determined by spectroscopy.


