phosphoric acid fuel cell
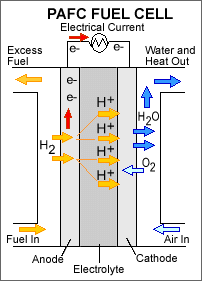
A phosphoric acid fuel cell consists of liquid phosphoric acid electrolyte sandwiched between an anode (negatively charged electrode) and a cathode (positively charged electrode). The processes that take place in the fuel cell are as follows: 1. Hydrogen fuel is channeled through field flow plates to the anode on one side of the fuel cell, while oxygen from the air is channeled to the cathode on the other side of the cell. 2. At the anode, a platinum catalyst causes the hydrogen to split into positive hydrogen ions (protons) and negatively charged electrons. 3. The phosphoric acid electrolyte allows only the positively charged ions to pass through it to the cathode. The negatively charged electrons must travel along an external circuit to the cathode, creating an electrical current. 4. At the cathode, the electrons and positively charged hydrogen ions combine with oxygen to form water, which flows out of the cell.
Phosphoric acid fuel cells (PAFCs) are fuel cells that use liquid phosphoric acid as an electrolyte – the acid is contained in a Teflon-bonded silicon carbide matrix – and porous carbon electrodes containing a platinum catalyst. The chemical reactions that take place in the cell are shown in the diagram to the right.
The phosphoric acid fuel cell is considered the "first generation" of modern fuel cells. It is one of the most mature cell types and the first to be used commercially. This type of fuel cell is typically used for stationary power generation, but some PAFCs have been used to power large vehicles such as city buses.
PAFCs are more tolerant of impurities in fossil fuels that have been reformed into hydrogen than PEM cells, which are easily "poisoned" by carbon monoxide because carbon monoxide binds to the platinum catalyst at the anode, decreasing the fuel cell's efficiency. They are 85% efficient when used for the cogeneration of electricity and heat but less efficient at generating electricity alone (37%–42%). This is only slightly more efficient than combustion-based power plants, which typically operate at 33%–35% efficiency. PAFCs are also less powerful than other fuel cells, given the same weight and volume. As a result, these fuel cells are typically large and heavy. PAFCs are also expensive. Like PEM fuel cells, PAFCs require an expensive platinum catalyst, which raises the cost of the fuel cell. A typical phosphoric acid fuel cell costs between $4,000 and $4,500 per kilowatt to operate.
