NAS battery
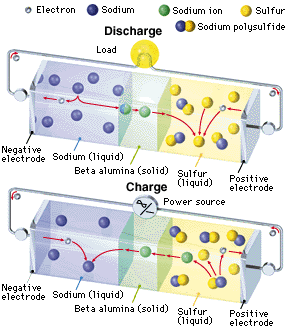
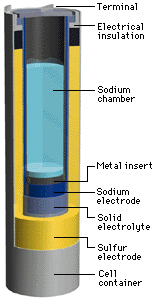
An NAS battery is a type of battery that uses sodium (Na) at the negative electrode (cathode), sulfur at the positive electrode (anode), and beta alumina (aluminum oxide) as a solid electrolyte. During the discharge phase, molten metallic sodium at the core acts as the anode, separated by a beta alumina cylinder from a sulfur container made from an inert metal acting as the cathode. The sulfur is absorbed in a carbon sponge. Alumina is a good conductor of Na ions but a bad conductor of electrons, avoiding self discharge. When Na gives off an electron, the Na+ ion migrates to the sulfur container. The electron travels through the molten Na to the contact and through the electric load to the sulfur container. Here, the electron reacts with S to form S-, which then forms sodium polysulfide. As the cell discharges the sodium level drops. During the charging phase the reverse process takes place.
The cell is usually made in a tall cylindrical configuration. The entire cell is enclosed by an inert metal container and sealed at the top with an airtight alumina lid. The cell becomes more economical with increasing size. In commercial applications the cells are arranged in blocks for better conservation of heat. Once running, the heat produced by charging and discharging cycles is enough to maintain operating temperatures and no external source is required.
An NAS battery has a high energy density and high efficiency of charge/discharge, and is made from inexpensive, non-toxic materials. However, the operating temperature of 300-350 °C and the highly corrosive nature of sodium make it suitable only for large-scale non-mobile applications. One such application is the storage of energy storage, during off-peak times, in the electric grid.
 |
