chlorofluorocarbons
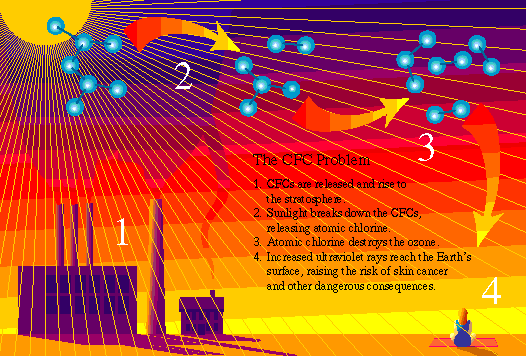
Chlorofluorocarbons (CFCs) are a family of chemicals composed primarily of carbon, hydrogen, chlorine, and fluorine; CFCs are used mainly as refrigerants for freezers, refrigerators, and air-conditioning units, and as industrial cleansing solvents primarily in the high-tech industry. They are also used as an expansion agent in the manufacture of isocyanurate thermal insulation, and some extruded polystyrene foam used for thermal insulation, and some extruded polystyrene foam used for thermal insulation products, and food service containers.
CFCs' ability to destroy stratospheric ozone through catalytic cycles is contributing to the depletion of ozone worldwide. Because CFCs are such stable molecules, they do not react easily with other chemicals in the lower atmosphere. One of the few forces that can break up CFC molecules is ultraviolet radiation, however the ozone layer protects the CFCs from ultraviolet radiation in the lower atmosphere. CFC molecules are then able to migrate intact into the stratosphere, where the molecules are bombarded by ultraviolet rays, causing the CFCs to break up and release their chlorine atoms. The released chlorine atoms participate in ozone destruction, with a single atom of chlorine able to destroy ozone molecules over and over again.
International attention to CFCs resulted in a meeting of diplomats from around the world in Montreal in 1987. They forged a treaty that called for drastic reductions in the production of CFCs. In 1990, diplomats met in London and voted to significantly strengthen the Montreal Protocol by calling for a complete elimination of CFCs by the year 2000.
