calorimetry
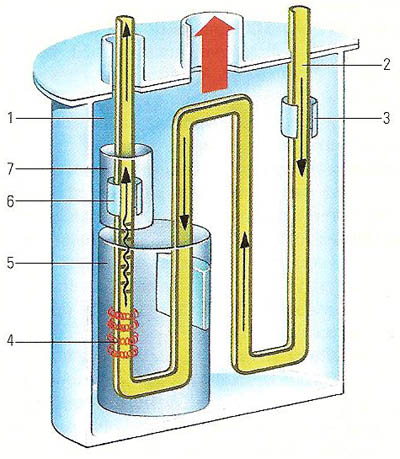
In a high-pressure flow calorimeter, the apparatus is contained in a vacuum (1) for insulation. A constant flow of liquid or gas enters the calorimeter (2). A platinum resistance thermometer (3) measures the temperature of the substance on entry. A heater (4) puts a known amount of energy into the liquid or gas inside a radiation shield (5) which further lessens any dispersion of energy. The change in temperature is measured by a second thermometer (6) again shielded (7).
Calorimetry is the measurement of the heat changes associated with chemical reactions and physical processes. This is done using various types of calorimeter.
The water calorimeter is a thermally insulated metal cup of known thermal properties containing a known mass of water. When a reaction is carried out in the vessel, any heat liberated or absorbed is taken or given up by the water, the heat changes occurring being monitored by means of a thermometer dipped in the water.
Heats of combustion are measured using a bomb calorimeter in which the reaction is carried out in an enclosed, pressurized chamber immersed in the water. Other types of apparatus used to measure specific heats and latent heats include the ice calorimeter and steam calorimeter.
See also thermochemistry.


