discovery of the electron
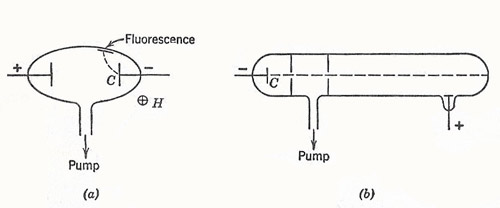
Figure 1. (a) Plücker's experiment. (b) A Crookes tube with a collimated beam of cathode rays.
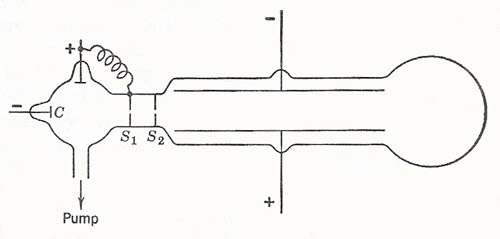
Figure 2. Schematic diagram of J. J. Thomson's e/m apparatus.
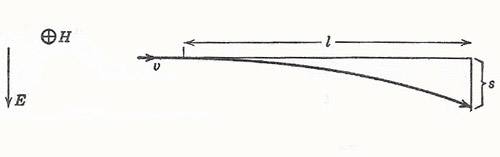
Figure 3. Deflection of an electron beam in crossed electric and magnetic fields.
The direct observation of this basic charge carrier was finally achieved in experiments with the low-pressure gas-discharge tube. This work had its beginning with the Geissler tube (1854), and many studies were made by Plücker (1858–1862). He found a green fluorescence near the cathode which could be moved about by a magnet (Figure 1a).
It was found that, if the pressure were made low enough, the fluorescence could be made to appear as a spot at the end of a suitably designed tube. The effect could be ascribed to "cathode rays" which traveled in straight lines through defining apertures (Figure 1b) but could be deflected by a magnet. During the period 1879–1885 Crookes made a series of experiments to show that the cathode rays were a stream of swift particles emitted from the cathode. In 1895 Perrin collected the rays in an insulated cup and showed that their sign was negative. Some people still thought that the cathode rays were something different from these negative particles, and were bothered by the apparent failure of the rays to be deflected by electric fields, though they were bent by magnetic fields.
In 1897 J. J. Thomson made the necessary exact measurements, which led to the identification of the rays. Using a pair of long parallel were something different from these negative particles, and were bothered by the apparent failure of the rays to be deflected by electric fields, though they were bent by magnetic fields. In 1897 J. J. Thomson made the necessary exact experiments, which led to the identification of the rays. Using a pair of long parallel plates, he demonstrated the deflection of cathode rays by an electric field (Figure 2). He also showed that this deflection could be canceled out by an appropriate magnetic field perpendicular to the electric field. By measuring the deflection in one field alone, and by knowing the values of the electric and magnetic fields, he could obtain quantitative information about charged particles in the rays.
For a potential difference V between plates a distance d apart, we have E = V/d
Let the length of path in the crossed electric and magnetic fields be l. Then the time of transit is given by t = l/v. The electric force is Ev, giving rise to a transverse acceleration Ee/m.
Therefore
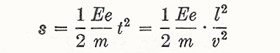 |
For balancing of electric and magnetic deflections,
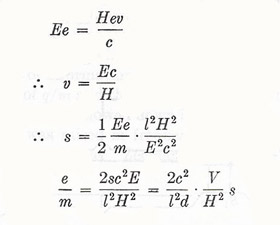 |
Thomson found e/m ≈ 1.7 × 107 emu/g (= 1.7 Am2/kg) under all conditions – independent of the nature of the gas in the cathode-ray tube and of the nature of the electrodes. This figure may be compared with the specific charge (i.e. ratio of charge to mass) concerned in the transfer of the lightest atoms (i.e. hydrogen) in electrolysis: q/MH = F/MH ≈ 104 emu/g.
Thus we must have, in the discharge tube, carriers that are either lighter than hydrogen atoms, or more heavily charged, or perhaps both. These carriers must be constituents of all matter. Lenard (1900) showed that the carriers of photoelectric current had this same e/m value (≈ 1.2 × 107 emu/g).


