entropy
Entropy is a measure of the disorder or unavailability of useful energy within a closed system. More entropy means less energy available for doing work. German physicist Rudolf Clausius produced the first mathematical formulation of entropy in 1854. However, it was not until 1865 that he coined the term "entropy", which comes from the Greek entropia, meaning "transformation content".
For an illustration of entropy, consider what happens when an ice cube is placed in a glass of water. Initially the energy in the glass is very unevenly distributed with the warmer water molecules possessing more energy than the colder ice. Consequently, the system is in a state of low entropy, with much of its energy available the ice. But once the system has achieved equilibrium, with the ice melted and thermal energy distributed randomly throughout the glass, the system is in a state of high entropy and its energy is unavailable for work.
This example also illustrates the second law of thermodynamics, which states that the entropy of any isolated system, one that exchanges neither matter nor energy with the outside world, always tends to increase. Without outside energy inputs, every system tends toward greater equilibrium, randomness, and disorder. Since the universe as a whole is an isolated system, it is steadily approaching a state of maximum entropy, at which point all its available energy will be spent.
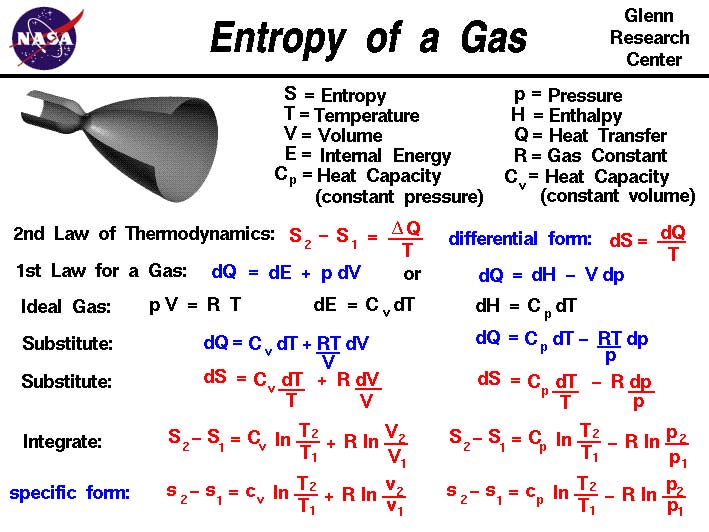
When a system undergoes a reversible change the entropy changes by an amount equal to the energy transferred to the system by heat divided by the thermodynamic temperature at which this occurs (i.e., the infinitesimal entropy change δS when a quantity of heat δQ is transferred at absolute temperature T is defined as δS = δQ/T).
Another way to think of entropy is the number of rearrangements of the ingredients of a system that leave its overall appearance intact, or alternatively as the amount of information about the microscopic motion of the atoms making up the system which is not determined by a description of the macroscopic state of that system.
Any change taking place in a system which results in an increase in entropy has a positive entropy change (ΔS). Most spontaneous thermodynamic processes are accompanied by an increase in entropy. Entropy has units of joules per degree K per mole.
See also enthalpy.


