Fischer-Tropsch reaction
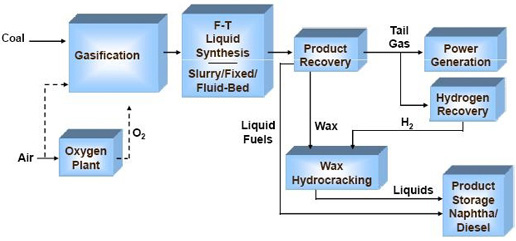
A simplified Fischer-Tropsch synthesis-based production scheme.
The Fischer-Tropsch reaction is a way of making organic molecules, especially long-chain functionalized aliphatic hydrocarbons, from carbon monoxide (CO) and hydrogen (H2) in the presence of a catalyst; similar processing can occur on a grain surface. It is named after F. Fischer and H. Tropsch, the German coal researchers who discovered it in 1923.
The reaction can be written in a simplified form as:
nCO + (2n+1)H2 → CnH(2n+2)+ nH2O,
where n is an integer.
For n = 1, the reaction represents the formation of methane, which in most applications is considered an undesirable byproduct. The Fischer-Tropsch process conditions are usually chosen to maximize the formation of higher molecular weight hydrocarbon liquid fuels which are higher value products.
Depending on the catalyst, temperature, and type of process employed, hydrocarbons ranging from methane to higher molecular paraffins and olefins can be obtained. Small amounts of low molecular weight oxygenates (e.g., alcohol and organic acids) are also formed. Catalysts considered for Fischer-Tropsch synthesis are based on transition metals of iron, cobalt, nickel and ruthenium.
The Fischer-Tropsch synthesis reaction, in theory, is a condensation polymerization reaction of CO. Its products obey a well-defined molecular weight distribution according to a relationship known as Shultz-Flory distribution.


