lime kiln
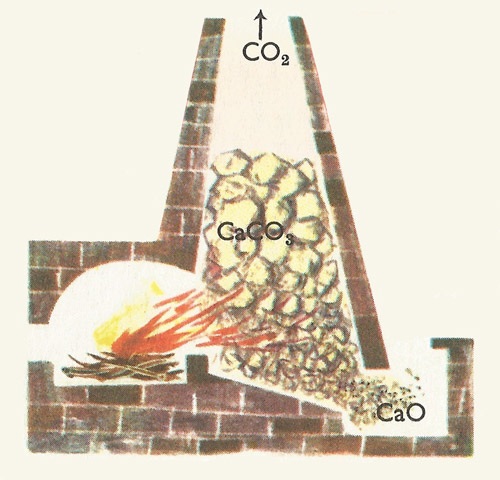
A lime kiln is a tall cylindrical furnace in which quarried limestone is heated with coke to a temperature of 1,000°C. Under the influence of this intense heat the calcium carbonate, which is a chemical compound of the metal calcium with two non-metals, carbon and oxygen decomposes. Some of the oxygen, together with the carbon, is driven off as the gas carbon dioxide and escapes from the top of the furnace, leaving behind quicklime, or calcium oxide, a compound of calcium with the remaining oxygen.
We can write the chemical equation for these changes like this:
 |
The older type of lime kiln has given way to huge rotating kilns heated by gas; these are more efficient.
Quicklime emerges from the old type of kiln as hard, white lumps and is sold to builders for the making of mortar and certain types of plaster. However, before the builder can use the quicklime has to be slaked. It is put in the ground and water is thrown on to it; it becomes very hot, and the lumps crumble to a fine white powder called slaked lime. The water has in fact combined chemically with the calcium oxide to form calcium hydroxide. Here is the chemical equation for this change:
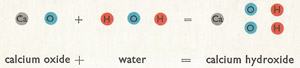 |
Mortar is made from slaked lime, sand, and sometimes, in addition, cement.
Slaked lime, calcium hydroxide, can easily be converted back into calcium carbonate by long exposure to the air. This is because it can combine with the calcium dioxide in the air, and indeed, part of the changes involved in the setting of mortar and plaster involve this reconversion of the lime to carbonate.
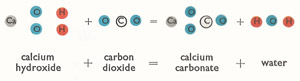 |
Both limestone and lime are weak alkalis and are used in agriculture to correct excess soil acidity and improve fertility.
Lime is used in enormous quantities in the manufacture of cement, which is obtained by heating lime with certain types of clay in huge rotary kilns. In some cement-making processes ground limestone is used in place of lime.
A piece of quicklime heated in a very hot gas flame glows with an intense white light. Before the invention of the electric light this property of lime was used in the limelight, a bright light for theatrical spotlights and projectors. Hence our expression 'in the limelight'.


