foundations of science in the 20th century

Figure 1. Faraday's "ring" was constructed for a central experiment in the work of Michael Faraday and the nature of electromagnetism in London in 1831. He knew that electricity flowing along a wire had an associated magnetic field and he argued that a magnet should create (by induction) an electric current in a wire placed near it. He showed that this could happen if the magnet was moving, and with this apparatus discovered self induction.
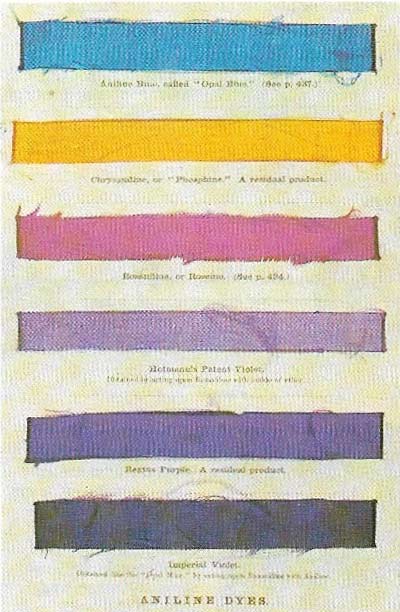
Figure 2. In the middle of the 19th century William Henry Perkin (1838–1907), in his laboratory, carried out research in organic chemistry and in particular into quinine and a substance derived from the coal-tar product aniline. By chance this led him to discover a mauve dye. Previously purple colours could be produced only from expensive natural product. Other aniline dyes followed - some early examples are shown from the Popular Science Review (1864).

Figure 3. Gregor Mendel was dissatisfied with current explanations of how the many different changes in, and varieties of, living things occurred. In the 1860s he began experiments in crossbreeding peas and found the existence of dominant and recessive characteristics (now called genes). This crossing tall and dwarf types give him a tall hybrid, not one half as tall, as current theory predicted. Tallness was the dominant characteristic. The next generation gave a quarter that was short; the recessive characteristic (dwarfness) had returned. Continued breeding showed the dwarf strain interbred as dwarfs. Mendel showed how proportions changed in later generations.

Figure 4. James Dewar (1842–1923) could not have given this demonstration of pouring liquid hydrogen without the 19th century's work on thermodynamics. The law of the conservation of energy and the identifying of heat as energy were vital advances. The idea that the heat of an object depends on the movement of its molecules led to the concept of absolute zero and is basic to the 20th century idea of matter.
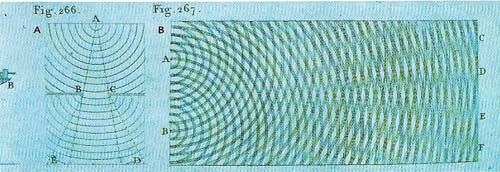
Figure 5. From 1800 to 1809 Thomas Young revived the wave theory of light, which opposed Newton's particle theory. In the 17th century the Dutch physicist Huygens suggested light was due to waves pushing outwards (longitudinal waves) from a source; Young however used up-and-down (transverse) wave, illustrated in his Course of Lectures on Natural Philosophy and the Mechanical Arts (1807). Interference patterns were explained by the wave theory of light.
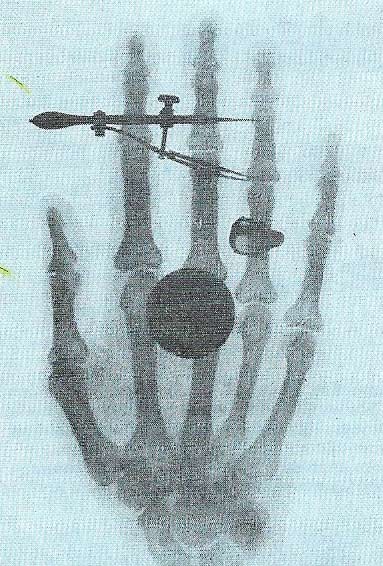
Figure 6. In 1896 it was discovered that uranium constantly emitted rays more penetrating than X-rays; such rays also made gases conduct electricity. This field was next studied by Marie and Pierre Curie. They found that other heavy substances emitted such rays (gamma rays). They analysed pitchblende, a uranium compound, and found it contained polonium and radium, the latter being a strong emitter. They realized the emission must be caused by some behaviour of the atoms in the materials; they called this behavior "radioactivity". The illustration shows Marie Curie's hand photographed using a gamma-ray source.

Figure 7. Albert Einstein's paper in 1905 on special relativity showed that not all physical quantities are capable of absolute measurement, but are relative to the same frame of reference. This did not take account of gravitation. But in 1915 he published his general theory of relativity which extended the idea to include gravitation and accelerated motion. Einstein's theory profoundly affected the outlook of modern science and the natural world.

Figure 8. The University of Gottingen, famous for its mathematics and physics facilities, produced some of the leaders in the massive expansion of physical science studies in the late 19th century Germany.

Figure 9. J. J. Thomson was director of the Cavendish Laboratory at Cambridge. With a brilliant research student, Ernest Rutherford, he found that when a rarified gas was bombarded by X-rays it became able to conduct electricity. From this Thompson was led to consider the nature of cathode rays, and this led him to discover that they were composed of electrons, the first subatomic particles, thus proving that atoms are not the smallest unit of matter.
Many fields of science in the nineteenth century seemed to be marked by orthodox progress – that is, a series of discoveries that could be fitted into the existing view of nature. Yet, in retrospect some of these discoveries were to lead to fundamental changes in the scientific picture. The new fields of thermodynamics and electromagnetism suggested new concepts of energy, and the mathematical work of the German Karl Gauss (1777–1855) and Georg Riemann (1826–1866), although purely academic in the 1850s was by the 1920s used as a means of describing the very nature of space.
Biology and medicine
The biological world also seemed straightforward until it was upset by Charles Darwin (1809–1882) and Gregor Mendel (1822–1884) (Fig 3). When Darwin produced his Origin of Species in 1859, placing man among the animals in an evolutionary process that worked by natural selection, a storm of controversy broke that slid not completely subside for more than a century. Mendel's work in the 1860s on inheritance factors went unnoticed at the time but in the 1900s was to help lay the foundations of genetics.
Medical science also progressed; Claude Bernard (1813–1878) studied the chemical properties of the digestive system and the treatment of infection improved with the new bacteriological ideas of Louis Pasteur (1822–1895). These studies, together with the introduction of antiseptics and anesthetics, were to lead to advances in surgery and in the understanding of new ways to combat disease. Medical scientists also began to explore the realms of the mind, virtually uncharted until the late nineteenth century, and in the work of Sigmund Freud (1856–1939) the foundations of psychoanalysis were laid and the important concept of the unconscious introduced.
 |
| This illustration of Louis Pasteur is from a cartoon published in Vanity Fair in 1887. Pasteur worked on fermentation, on the souring of milk, on putrefaction and then on a disease in silkworms. He showed that all were due to the presence of microorganisms proved that these were airborne. He then studied other animal diseases and devised a method of immunisation by inoculating a toxin to raise the host's resistance to more virulent types of the organism. |
 |
| Sigmund Freud, a Viennese, developed the theory of the "psyche" at first through the use of hypnosis in the treatment of hysteria and later through the technique of free association. Both probed the unconscious and its power to affect conduct. He also stressed the sexual motivation behind much human behaviour. Freud's ideas, which included the analysis of dreams, were taken up and modified by others, particularly Carl Jung. |
The atomic theory
Chemistry finally broke its remaining ties with alchemy and its mysticism, becoming a true practical and scientific study according to the principles laid down by Antoine Lavoisier (1743–1794). Its central advance was the atomic theory. Introduced in its modern form at the beginning of the nineteenth century by John Dalton (1766–1844), the theory propounded the view that all chemical changes were merely rearrangments of individual and indestructible atoms.
It took some time before the theory was accepted, since a considerable amount of independent evidence was deemed necessary, yet in the work of Amadeo Avogadro (1776–1856), Stanislao Cannizzaro (1826–1910), and Rins Berzelius (1799–1848), the desired correlations and experimental proofs were found and the theory of the atomic nature of matter became established. Other discoveries followed not least in the field of organic chemistry. These advances were due especially to the work of Justus von Liebig (1803–1873) on agricultural chemistry and fertilizers, and Friedrich Kekule (1829–1896) who discovered the ring-like structure of the atoms in a molecule of benzene and similar compounds. This has led to the development of plastics and many other petrochemical products, to the development of modern synthetic drugs and explosives and to a better understanding of the chemistry of food and of all living things.
In physics important advances were made in the understanding of electricity. This began in 1800 with the construction of a cell or battery – the "voltaic pile" – by Alessandro Volta (1745–1827), by means of which a continuous flow of electricity was obtained for the first time. There was also research by others, especially Humphry Davy (1778–1829), on the chemical effects of electricity and Michael Faraday (Fig 1) on the connections between electricity and magnetism. His ideas were taken up and carried further by James Clerk Maxwell (1831–1879) who studied the mathematical properties of the electromagnetic field, and predicted the existence of types of electromagnetic radiation other than light.
Experiments with light
Maxwell used the theory that light moves in waves, which had been developed by the experiments in interferometry by Thomas Young (1773–1829) (Figure 5). Interest in the nature of life was intensified by the invention of spectroscopy, too, by Joseph von Fraunhofer (1787–1826) and later developed by Gustav Kirchhoff (1824–1887). This proved to be a delicate means of chemical analysis and it soon became a revolutionary tool of astronomical research. In the 19th century it was generally assumed that light travelled through an invisible substance known as the ether, although no proof of its existence was available. An experiment conducted in 1887 by A. Michelson (1852–1931) and E. W. Morley (1838–1923) indicated that this substance did not exist. This experiment left physics in a state of confusion until the publication of the special theory of relativity by Albert Einstein (1879–1955) in 1905 (Figure 7).
In other fields, too, new studies at the end of the nineteenth century brought important breakthroughs. Work on the discharge of electricity through gases, in particular, led to astonishing results. William Crookes (1832–1919) discovered cathode rays and J. J. Thomson (1856–1940) (Figure 9) the electron – together with the quantum theory of Max Planck (1858–1947), the cornerstone of atomic and nuclear physics.
