MICROMACHINES AND NANOTECHNOLOGY: The Amazing New World of the Ultrasmall - 4. How Small Can You Get?
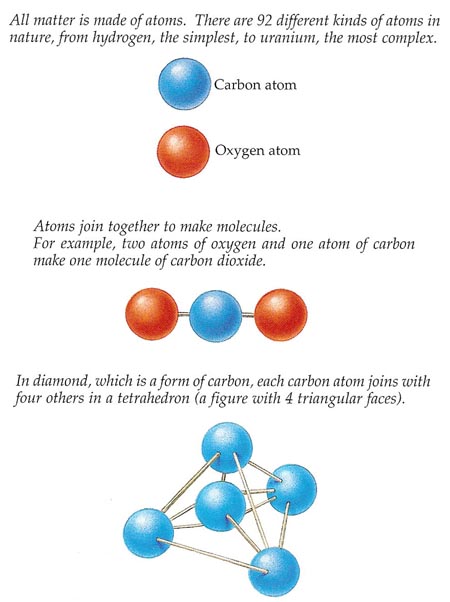
Figure 1. Atoms and molecules.

Figure 2. The flat faces of this crystal of quartz are caused by the atoms inside being lined up in a regular way.
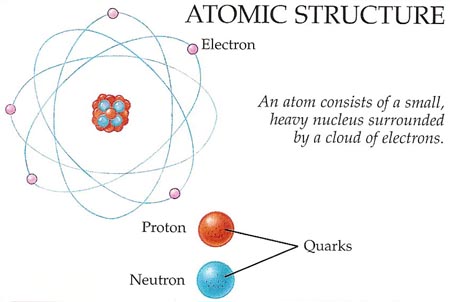
Figure 3. Inside the atom.
Take a grain of sugar and break it in half. Now take one of these smaller grains and break it in half again. Suppose you have the means to keep on splitting the bits of sugar exactly two, again and again, even after they are much too tiny to be seen. Eventually you will reach a limit. You will end up with a fragment of sugar so small that if you divide it just one more time it will no longer be sugar. This smallest possible piece of sugar is called a molecule (see Figure 1).
Most natural and human-made substances are made of molecules. Molecules, in turn, are built of even smaller particles called atoms. One molecule of household sugar (sucrose) consists of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen, joined together. A simpler molecule, such as that of water, contains just 2 atoms of hydrogen and 1 of oxygen.
Some substances, such as the precious metal gold and the lightweight gas helium, consist of atoms that are not linked together to make molecules. So, with the right equipment, you could pluck a single atom of gold out of a gold ring or a single atom of helium out of a helium-filled balloon.
Sizing Up Atoms
It is very hard to visualize how small atoms really are. Ten million of them placed in a line would fit across the period at the end of this sentence. Put another way, if everything in the world were blown up in size so that a single atom were as big as this period, then the smallest letters on this page would be about 50 miles high!
Even the smallest of ordinary things is made up of unimaginably huge numbers of atoms. A single pin, for instance, contains roughly a million times as many atoms as there human beings on Earth.
An ordinary hand-held machine, such as a hair dryer, contains about as many atoms as there are grains of sand on all the world's beaches. When a machine like this is built, no one knows or cares where each individual atom inside is located. It might seem beyond belief that people could eventually learn how to build anything one atom at a time.
Nature's Building Blocks
Atoms are incredibly small, but we know without a doubt that it is possible to put them together, one by one, in a very orderly way. We know this because nature does it all the time. The perfectly flat sides and regular shapes of crystals are due to the precise arrangement of atoms inside the crystals (see Figure 2). As a crystal forms, individual atoms add on to the growing structure like people sitting down, one at a time, from front to back, on the neat rows of evenly spaced seats in a theater.
The same orderly growth of structures takes place within living things. A human being is not a confused heap of atoms and molecules. As a baby grows, it does so according to a definite pattern. Like all the different kinds of animals and plants, a human being assembles itself in an organized way from individual atoms and molecules.
If our bodies can control their growth at the atomic and molecular level, then perhaps scientists and engineers can learn to do the trick by artificial means. This suggestion was first made by the brilliant American scientist Richard Feynman, in a speech in 1959. Feynman said: "Consider the final question as to whether, ultimately – in the great future – we can arrange atoms the way we want: the very atoms, all the way down! What would happen if we could arrange atoms one by one, the way we want them!"
Nanotechnology Is Born
If we could find a way to put atoms together, one at a time, exactly as wanted, we could build almost anything at all. We could make a robot spacecraft that was smaller than a pollen grain. We could mass-produce a billion of these tiny explorers and scatter them throughout space, just as real pollen is blown about on the wind. In the remote future, marvelous things like these may be possible. But first, researchers will have to become experts at working with atoms.
Some years after Richard Feynman made his speech about building with atoms, the author Eric Drexler coined the name nanotechnology. People have been familiar for a long time with words beginning with MICRO. There are microscopes, microbes (tiny living things), and microchips, to name a few. Micro comes from the ancient Greek micros, which means "small." In science, micro also stands for 1-millionth. A micrometer, for instance, is 1-millionth of a meter.
For defining even smaller measurements, scientists use the prefix nano. This comes from the Greek nanos, meaning "dwarf." A NANOMETER is a thousand times smaller than a micrometer, or 1-billionth of a meter.
Because micro was a term already used to refer to anything microscopic, nano is a good name to describe the possibility of working at even
smaller scales. Nanotechnology, then, is the technique of building machines
and other structures from individual atoms and molecules.
| Inside the Atom
Given enough energy, atoms themselves can be split apart into even smaller particles. At the center of an atom is the nucleus – a cluster of particles made up of protons and neutrons (see Figure 3). The proton has a single positive charge, while the neutron is electrically neutral. Around the nucleus is a cloud of even smaller particles, called electrons, each of which has a negative charge that is equal in size and opposite in sign to the charge on the proton. Because, in every atom, the number of protons is the same as the number of electrons, atoms have no overall charge.
Electrons are believed to be pointlike specks of matter that cannot be broken apart any further. Protons and neutrons, however, are each thought to be a kind of bag containing three smaller particles known as quarks.
Scientists are now learning how to control the movements of individual electrons or small groups of electrons. This knowledge may eventually allow them to create new kinds of computer memories capable of storing fantastic amounts of information. For the purposes of nanotechnology, however, the smallest possible building blocks wil be atoms and molecules. |

