activation energy
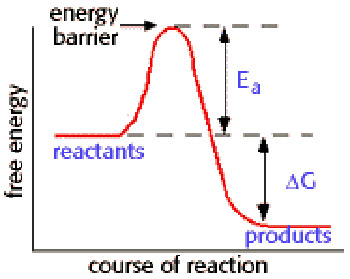
Activation energy is the minimum energy needed for a specific chemical reaction to take place, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence of the reaction rate. A catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction.
As the accompanying diagram shows, the activation energy, Ea, is distinct from the free energy difference, ΔG, between the reactants and products.


