cohesion
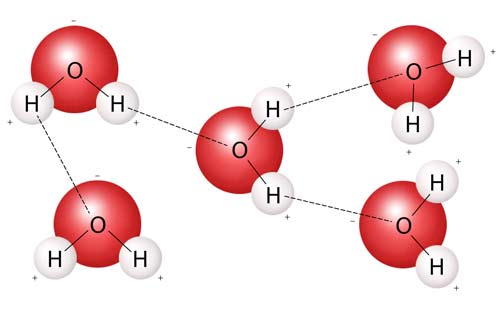
The strong polar bond between water molecules creates water cohesion.
Cohesion is the tendency of different parts of a substance to hold together. Cohesion is due to forces acting between its molecules: a molecule will repel one close to it but attract one that is farther away; somewhere between these is a position where work must be done to either separate the molecules or push them together. This situation results in both cohesion and adhesion. Cohesion is strongest in a solid, less strong in a liquid, and least strong in a gas; its strength decreases with rise in temperature.


