diamond
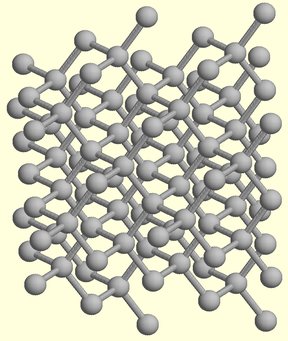
Figure 1. Molecular structure of diamond.
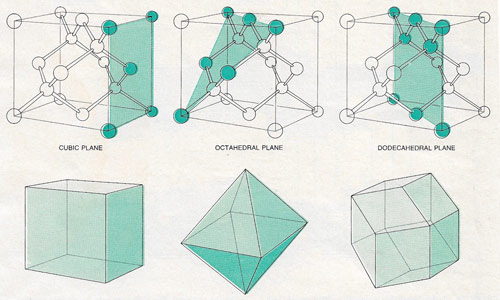
Figure 2. The most common faces of a diamond crystal are parallel to one of three kinds of plane in the unit cell. The cubic planes (left), of which there are six, are the faces of the unit cell itself. The octahedral planes (center) extend from the diagonal of a face to an opposite corner; eight such planes can be produced by reflection and rotation. The dodecahedral planes (right) are diagonals of the unit cube; there are 12, two for each possible orientation of the cube. Below the crystal planes is the form generated when the crystal grows exclusively on each kind of plane; real diamonds often have facets representing growth in each of the regime.

Figure 3. Hypothetical star with diamond core.
Diamond is an allotrope (see allotropy), or structural form, of carbon. Diamond is the hardest substance known, with a Mohs hardness of 10, and the least compressible. It is a better conductor of heat at room temperature than any other material, and when completely pure is transparent. These extreme properties make diamond technologically very useful. Its hardness makes it useful as a cutting tool in industry and surgery, and because of its excellent heat conductivity it is used as a heat sink to cool electronic components rapidly. These applications are a direct result of the internal structure of diamond, coupled with the fact that the carbon atoms in diamond are more closely packed than the atoms in any other material.
The atoms in a crystal of diamond are bonded into one giant molecule. Each carbon atom is covalently bonded to four other carbon atoms, and so each carbon atom has four nearest neighbors. That is, its coordination number is 4. When just four bonding pairs of electrons surround an atom, they are arranged tetrahedrally. This is the case with diamond. All outer electrons of the carbon atoms are involved in the formation of covalent bonds. There is no possibility of delocalized or mobile electrons. As a consequence, diamond cannot conduct electricity.
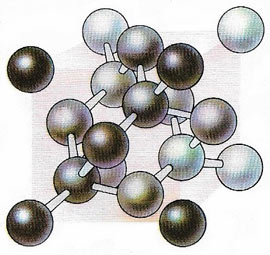 |
| Ina diamond any one carbon atom is covalently bonded to four others, orientated at the corners of a tetrahedron. This strain-free covalent configuration accounts for the diamond's hardness.
|
Diamond burns when heated in air to 900°C; in an inert atmosphere it reverts to graphite slowly at 1,000°C, rapidly at 1,700°C.
Natural diamonds occur in some ancient volcanic "pipes," such as those in South Africa, Tanzania, and in the United States at Murfreesboro, Arkansas, and have been recovered from the ocean floor off the Cape of Good Hope. Microscopic diamonds have also been found in some meteorites.
Diamond stars
The interior of some white dwarf stars is believed to consist of crystalline carbon – in effect, these would be diamonds as big as small planets (Figure 3). In 2004, astronomers announced that measurements they had made of BPM 37093, a white dwarf lying some 50 light-years from Earth in the constellation Centaurus, probably has a diamond core about 4,000 kilometers in diameter. BMP 37093 pulsates regularly. By studying these pulsations, the researchers were able to reveal the hidden interior of the white dwarf, just as seismograph measurements of earthquakes allow geologists to learn about the interior of Earth.
Diamonds in meteorites
Diamonds have been found inside meteorites. Some of these diamonds were formed by shock pressure during violent impacts between asteroids in the asteroid belt. Shock-formed diamonds tend to be very cracked and measure one or two millimeters in size.
Another type of diamond in meteorites occurs as tiny crystals measuring only a few nanometers across. They contain an isotope of xenon that is very rare on Earth but common in supernova remnants, suggesting that this is where they formed. Nanodiamonds are also surprisingly abundant, making up about 3% of the carbon in meteorites in which they occur. If the same is true of carbon in interstellar space, just a gram of the dust and gas in an interstellar cloud could contain as many as 10,000 trillion nanodiamonds.
Black diamonds
So-called black diamonds, or carbonado, may be also have come from space. Black diamonds are only found in Brazil and the Central African Republic and, unlike other diamonds, are made of millions of diamond crystals stuck together. They are porous, too, which is puzzling because it would have been difficult for gas to become trapped in rocks at the depths at which terrestrial diamonds were formed – about 200 kilometers below the surface. In a paper published in 2007, Stephen Haggerty, a geologist at Florida International University, proposes that the black diamonds arrived from space in a kilometer-sized rock between 2.6 billion and 3.8 billion years ago. At that time, South America and Africa were one land mass, which could account for the diamonds showing up on two continents today.


