distillation
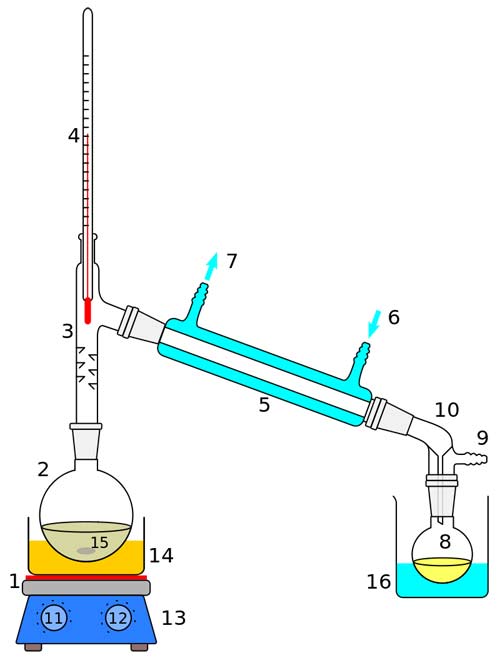
Laboratory display of distillation: 1: A heat source 2: Round bottomed flask 3: Still head 4: Thermometer/Boiling point temperature 5: Condenser 6: Cooling water in 7: Cooling water out 8: Distillate/receiving flask 9: Vacuum/gas inlet 10: Still receiver 11: Heat control 12: Stirrer speed control 13: Stirrer/heat plate 14: Heating (Oil/sand) bath 15: Stirring mechanism (not shown) e.g. boiling chips or mechanical stirrer 16: Cooling bath.
Distillation is a process in which substances are vaporized and then condensed by cooling. Distillation was probably first invented by the Alexandrian school and was used in alchemy, the still and the alembic being employed. It may be used to separate a volatile liquid from nonvolatile solids, as in the production of pure water from seawater, or from less volatile liquids, as in the distillation of liquid air to give oxygen, nitrogen, and the noble gases.
If the boiling points of the components differ greatly, simple distillation can be used: on gentle heating, the components distil over in order (the more volatile first) and the pure fractions are collected in different flasks. Mixtures of liquids of similar boiling points require fractionation for efficient separation. This technique employs multiple still heads and fractionating columns in which some of the vapor is condensed and returned to the still, equilibrating as it does so with the rising vapor. In effect, the mixture is redistilled several times; the number of theoretical simple distillations, or theoretical plates, represents the separating efficiency of the columns.
The theory of distillation is an aspect of phase equilibria studies. For ideal solutions, obeying Raoult's law, that vapor always contains a higher proportion than the liquid of the more volatile component; if this is not the case, an azeotropic mixture may be formed. When two immiscible liquids are distilled, they come over in the proportion of their vapor pressures at a temperature below the boiling point of either. This is utilized in steam distillation, in which superheated steam is passed into the still and comes over together with the volatile liquid. It is useful when normal distillation would require a temperature high enough to cause decomposition, as is vacuum distillation, in which the pressure reduction lowers the boiling points. A further refinement is molecular distillation, in which unstable molecules travel directly in a high vacuum to the condenser.


