evaporation
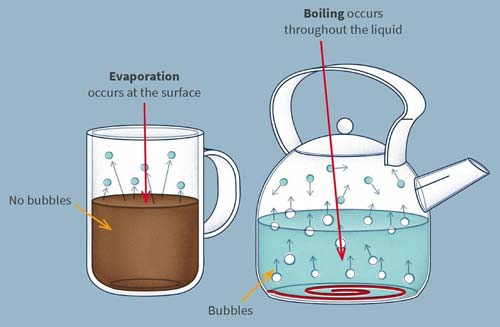
Evaporation is the escape of molecules from the surface of a liquid into the vapor state, without necessarily reaching the boiling point. Only those molecules with above-average energy are able to overcome the cohesive forces holding the liquid together and escape from the surface. Eventually all the molecules left in the liquid have become below-average energy; its temperature has now lower. In an enclosed space the pressure of the vapor above the surface eventually reaches a maximum called the saturated vapor pressure (SVP). This varies according to the substance concerned and, together with the rate of evaporation, increases with temperature, equaling atmospheric pressure at the liquid's boiling point.
Evaporation is used in concentrating solutions by evaporating off the solvent.


