half-cell
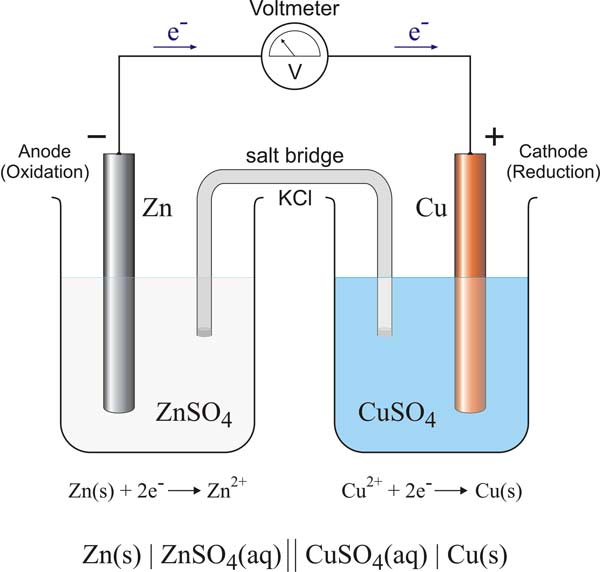
A half-cell is a part of galvanic cell in which oxidation or reduction of an element in contact with water or water solution one of its compounds takes place.
A half-cell is an electrode immersed in an ionic solution. A full electrolytic cell is formed of two half-cells – different solutions being separated by a membrane or conducting bridge that allows electricity to flow but prevents mixing. See also electrode potential.


