heat of vaporization
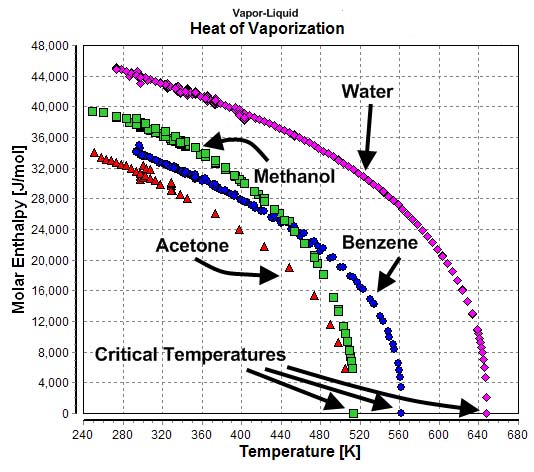
Temperature-dependency of the heats of vaporization of water, methanol, benzene, and acetone.
The heat of vaporization is the amount of energy needed to change a liquid into a vapor once it has reached its boiling point. Together with heat capacity, it is an important property in determining how effectively a solvent can regulate the internal temperature of an organism. The fact that water has both a high heat of vaporization and a high heat capacity makes it ideal in this respect, and is one of the reasons it is so essential to life as we know it.


