heme
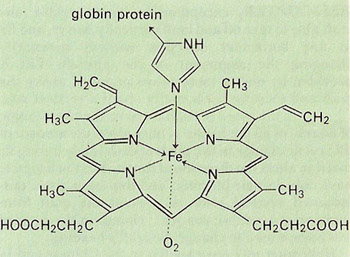
The heme unit of which there are four in each hemoglobin molecule is planar porphyrin structure, linked to the globin proteins which make up the bulk of the hemoglobin molecule via the amino acid histidine, part of which is shown here above the plane of the porphyrin ring. The histidine coordinates with the iron (II) atom of the heme, which also, in oxyhemoglobin, loosely binds the oxygen.
Heme, C34H32FeN4O4, is a subunit of the hemoglobin molecule. It consists of an organic part, called protoporphyrin IX, and an iron atom. The organic part is made up of four rings that are linked together to form a tetra structure. The iron atom is in the center of the four rings and binds to oxygen. Structurally it resembles hematin with the iron in the Fe(II) state and with no chlorine. Heme combines with the protein globin to form the blood pigment hemoglobin.
Heme serves as the prosthetic group of hemoglobin, of cytochromes, and of some peroxidases. The iron may be oxidized to Fe(III) during reactions involving cytochromes or peroxidases, i.e. it becomes hematin.


