latent heat
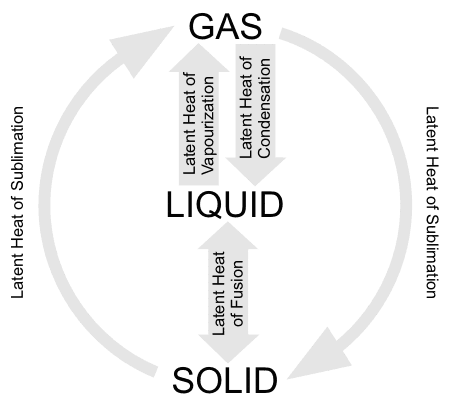
Latent heat is the quantity of heat required to bring about a change of state of a unit mass of a substance from solid to liquid (latent heat of fusion) or from liquid to gas (latent heat of vaporization or of condensation) or from solid to gas directly (latent heat of vaporization) without change of temperature (i.e., isothermally).
At the freezing point and boiling point of a substance, adding heat produces no rise in temperature until the change of state is complete. Energy required to effect the change of state is absorbed in the form of latent heat, and an equal amount of heat is liberated in the reverse process. In the case of ice changing to water at 0°C, for example, the heat energy absorbed during melting is used exclusively to overcome the intermolecular forces in the order ice structure; only when this is accomplished and further heat is added does the kinetic energy of the water molecules and, hence, the temperature of the water begin to rise above 0°C.


