lime water
Lime water is used as a test for carbon dioxide.
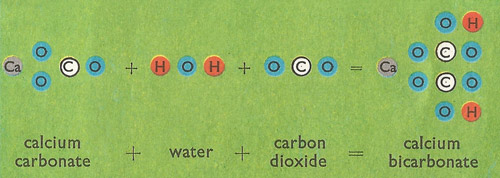
Lime water is a saturated solution of calcium hydroxide (slaked lime, Ca(OH)2). It is used to detect carbon dioxide (CO2), which forms a milky white precipitate of calcium carbonate (CaCO3) when bubbled through lime water. Excess carbon dioxide changes the calcium carbonate into calcium hydrogencarbonate (calcium carbonate, Ca(HCO3)2), which is soluble and so the white precipitate disappears.
Experiment with lime water
Take a straw and blow into a container of lime water: it will go cloudy. This is because the carbon dioxide in your breath has converted the dissolved calcium hydroxide into insoluble calcium carbonate, which separates out. If you go on blowing for a long time the cloudy suspension of calcium carbonate will become clear again. This is because the insoluble carbonate combines with more carbon dioxide to give calcium bicarbonate, which is soluble in water.


