molten carbonate fuel cell
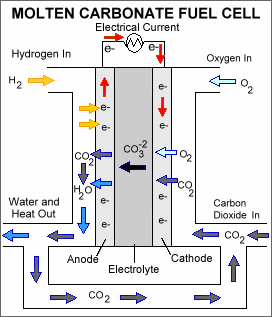
A molten carbonate fuel cell consists of an electrolyte, typically a molten carbonate salt mixture suspended in a ceramic matrix, sandwiched between an anode (negatively charged electrode) and a cathode (positively charged electrode). The processes that take place in the fuel cell are as follows: 1. Hydrogen fuel is channeled through field flow plates to the anode on one side of the fuel cell, while oxygen from the air, carbon dioxide, and electricity (electrons from the fuel cell circuit) are channeled to the cathode on the other side of the cell. 2. At the cathode, the oxygen, carbon dioxide, and electrons react to form positively charged oxygen ions and negatively charged carbonate ions. 3. The carbonate ions move through the electrolyte to the anode. 4. At the anode, a catalyst causes the hydrogen combine with the carbonate ions, forming water and carbon dioxide and releasing electrons. 5. The electrolyte does not allow the electrons to pass through it to the cathode, forcing them to flow through an external circuit to the cathode. This flow of electrons forms an electrical current. 6. The carbon dioxide formed at the anode is often recycled back to the cathode.
Molten carbonate fuel cells (MCFCs) are currently being developed for natural gas and coal-based power plants for electrical utility, industrial, and military applications. MCFCs are high-temperature fuel cells that use an electrolyte composed of a molten carbonate salt mixture suspended in a porous, chemically inert ceramic lithium aluminum oxide (LiAlO2) matrix. Because they operate at extremely high temperatures of 650°C (roughly 1,200°F) and above, non-precious metals can be used as catalysts at the anode and cathode, reducing costs.
Improved efficiency is another reason MCFCs offer significant cost reductions over phosphoric acid fuel cells (PAFCs). Molten carbonate fuel cells can reach efficiencies approaching 60%, considerably higher than the 37%–42% efficiencies of a phosphoric acid fuel cell plant. When the waste heat is captured and used, overall fuel efficiencies can be as high as 85%.
Unlike alkaline fuel cells, PAFCs, and polymer electrolyte membrane fuel cells, MCFCs do not require an external reformer to convert more energy-dense fuels to hydrogen. Due to the high temperatures at which MCFCs operate, these fuels are converted to hydrogen within the fuel cell itself by a process called internal reforming, which also reduces cost.
Molten carbonate fuel cells are not prone to carbon monoxide or carbon dioxide "poisoning" – they can even use carbon oxides as fuel – making them more attractive for fueling with gases made from coal. Because they are more resistant to impurities than other fuel cell types, scientists believe that they could even be capable of internal reforming of coal, assuming they can be made resistant to impurities such as sulfur and particulates that result from converting coal, a dirtier fossil fuel source than many others, into hydrogen.
The primary disadvantage of current MCFC technology is durability. The high temperatures at which these cells operate and the corrosive electrolyte used accelerate component breakdown and corrosion, decreasing cell life. Scientists are currently exploring corrosion-resistant materials for components as well as fuel cell designs that increase cell life without decreasing performance.
