octane
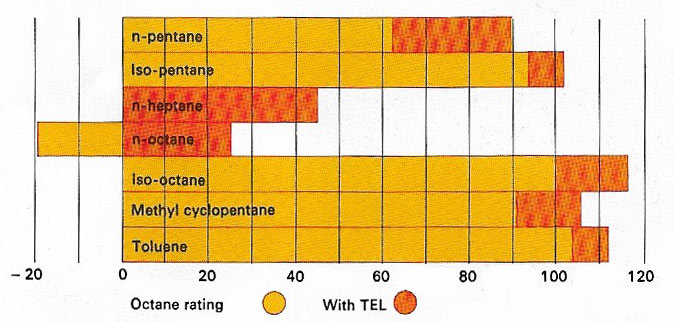
The octane number or rating of engine fuel – gasoline or petrol – is a measure of its efficiency for modern engines. Hydrocarbons with branched chains or cyclic structures are better than straight-chain compounds. All are improved by the addition of the organometallic compound tetraethyl lead (TEL), although this causes pollution.
Octane (C8H18) is a liquid alkane with 18 isomers that are constituents of gasoline. Normal octane occurs in petroleum; the branched isomers, which have high antiknock values, are made by alkylation.
The octane number of a gasoline is the percentage of isooctane (2,2,4-trimethylpentane) in the mixture of isooctane with n-heptane, which, in standard tests, knocks to the same degree as the gasoline. Antiknock additives and modern refining techniques can raise the octane number above 100.


