pH
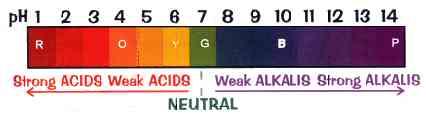
The pH scale, showing the color that Universal Indicator changes to for different pH values.
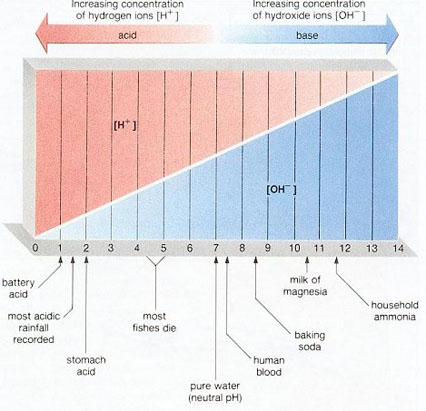
pH is a measure of the positive hydrogen ion (H+) concentration in water. pH equals the negative logarithm of the H+ concentration. Solutions with a high H+ concentration have a low pH (less than 7) and are said to be acidic, while those with a low H+ concentration have a high pH and are said to be basic or alkaline. pH values range from 1 for the strongest acids to 14 for the strongest alkalis. Pure water has a pH of 7. pH may be measured with an indicator or meter.
The pH (potential of hydrogen) scale was introduced (1909) by S. P. Sorensen (1868–1939).
A neutral pH near 7 is optimal for many biological processes, although some, such as the light reactions of photosynthesis, depend on pH gradients. In nature, pH can be high, such as in soda lakes or drying ponds, or as low as 0 and below. Organisms that live at either extreme do this by maintaining the near-neutral pH of their cytoplasm, i.e., the liquid and materials within their cells. See also acidophiles and alkaliphile.


