Soxhlet apparatus
Using the Soxhlet apparatus.
The Soxhlet apparatus.
Edible oils can be extracted from various vegetable sources, such as groundnuts and coconuts, by passing petroleum spirit over the crushed seeds. If the petroleum is cold only small quantities of the oil are recovered, while if it is too hot the oil molecules are oxidized and rendered useless. The temperature of the extraction process has, therefore, to be carefully controlled.
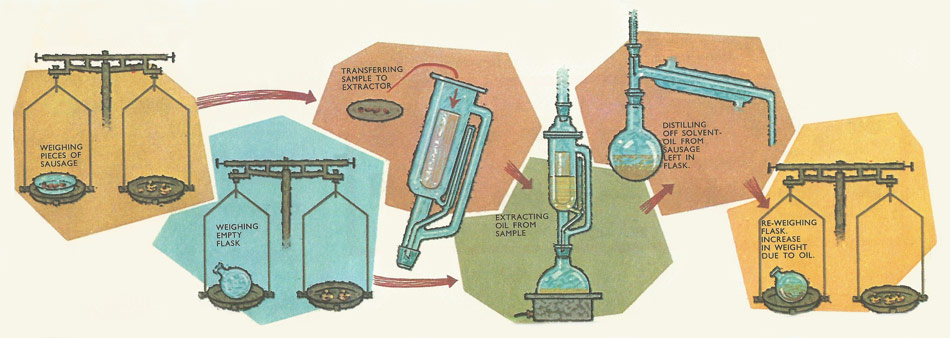
Before each batch of seed is accepted as a source of vegetable oil, samples must undergo careful tests. The proportion of oil in the seed is usually measured using the Soxhlet extraction apparatus. This same apparatus is also used in the determination of the fat content of manufactured foods such as sausages. Since the highest temperature which the sample experiences is that of the boiling solvent, the chemical composition of the oil is preserved. In this way the maximum amount of oil is extracted without spoiling it.
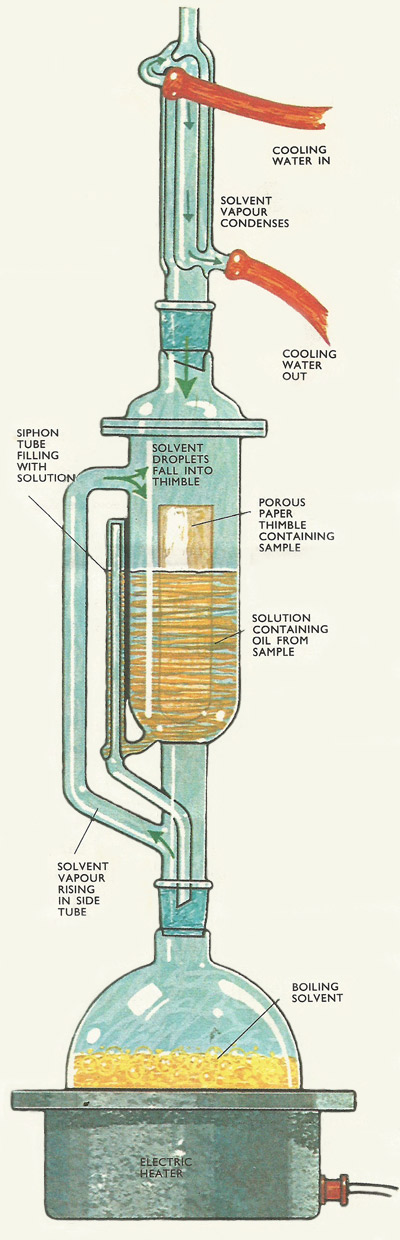
The Soxhlet apparatus consists of three units which fit together one above the other. At the base is a round bottomed flask in which the solvent is boiled. The extractor itself fits into the neck of the flask, while a condenser is attached to the top of the extractor. In all modern versions of the apparatus the three components are joined together by means of standard ground glass joints. These are virtually leak proof, so that no oil nor inflammable vapor is allowed to escape.
The solvent is poured into the flask while the sample, supported in a paper thimble, is placed in the main body of the extractor. The flask is usually warmed by an electric heating mantle. Vapor from the flask rises through the extractor's side tube and most of it enters the condenser. Condensate then drips down on to the sample in the body of the extractor.
At first there is not much liquid around the sample. At this stage the sample is warmed by the hot vapor of the solvent and this helps to loosen the solid particles (which in some instances are packed closely together). Then, gradually the extractor becomes filled with solvent, and as the liquid level rises around the sample, more and more of the oil dissolves in the solvent.
Eventually the liquid level reaches the top of the siphon tube. As soon as this siphon tube fills with solution, all the liquid in the extractor is quickly drained back into the round bottomed flask below.
As the liquid in the flask continues to boil all the while, there will be very little interruption in the supply of vapor to the condenser and the condensate to the extractor. And the condensate is always pure (or almost pure) solvent. Since the oil extracted by the solvent has a much higher boiling point it remains in the flask. This means that the maximum amount of oil can be extracted by each successive filling of the extractor.
When, eventually, all the oil has been extracted the solution is collected in the flask. Then all the solvent is removed by careful evaporation. The oil is left behind in the flask, so that any increase in weight of the flask is due to the soil. The proportion of oil in the sample is then calculated by comparing the weight of oil recovered with the total weight of the sample.


