spectrum
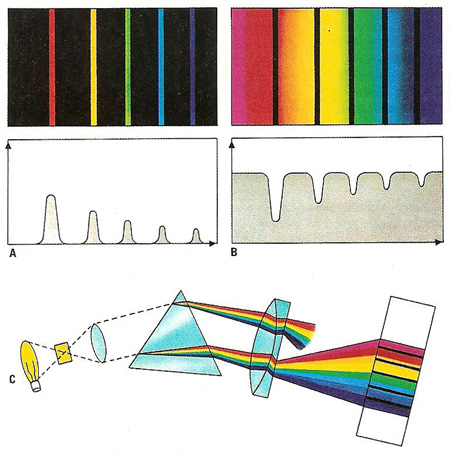
There are two fundamental types of line spectra: an emission spectrum and an absorption spectrum. An emission (A) spectrum (A) is the result of exciting a substance so that its electrons move to a higher energy. Photons are given out when the electrons fall back to their original state. Conversely, an absorption spectrum (B) is obtained when a photon is absorbed, raising the electrons of an atom to a higher level. A substance that emits light at a certain frequency absorbs light at the same frequency. When white light passes through a substance (C), an absorption spectrum can be seen – that is, the full spectrum (except for black lines) at the wavelengths the substance would emit if glowing alone.
In optics, a spectrum is a plot of the intensity of electromagnetic radiation radiation at different wavelengths; in the case of visible light, this is the familiar rainbow of colors. An object that emits radiation in a continuous range of colors is said to have a continuous spectrum. An object that emits radiation only at certain wavelengths is said to have emission lines; objects that absorb radiation only at certain wavelengths are said to have absorption lines. A good spectrum of a star, for example, reveals, among other things, its spectral type, radial velocity, and metallicity.
In quantum mechanics, a spectrum is the set of allowed energy levels of a particle or system. It is directly related to bright or dark lines in a spectrum of light produced by a prism.
In mathematics, a spectrum is the set of eigenvalues of a linear transformation. By historical coincidence, it is equivalent to the notion of a spectrum in quantum mechanics.


