catalytic converter
A catalytic converter is an anti-pollution device used in internal combustion engines. It consists of a bed of catalytic agents through which flow the gaseous exhaust of fuel combustion. Converters located in mufflers (silencers) reduce harmful unburned hydrocarbons and carbon monoxide. These converters are adversely affected by tetraethyl lead found in some types of gasoline.
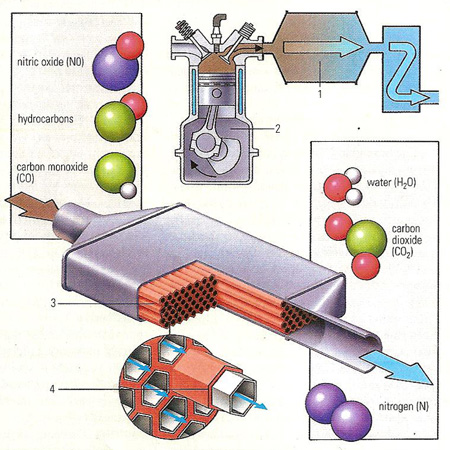
A catalytic converter (see diagram) is placed in the exhaust system (1) to reduce the pollution produced by combustion engines (2). It comprises a ceramic honeycomb structure (3), which maximizes the surface area of the converter, covered in catalysts – normally platinum and rhodium (4). As exhaust gases, primarily carbon monoxide, nitric oxide, and hydrocarbons from the cylinder, pass through the converter they react with the catalysts. The platinum and rhodium accelerate oxidation and reduction in the hot gases. The pollutants are oxidized into water, carbon dioxide, and nitrogen.


