BETWEEN FIRE AND ICE: The Science of Heat - 3. From Ice Cubes to Saunas
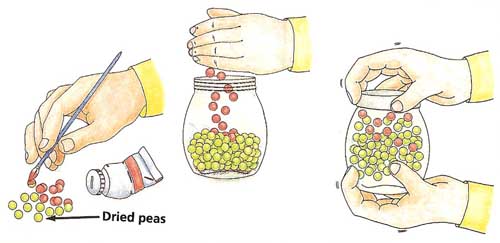
Figure 1. An experiment using peas as molecules.

Figure 2. A piece of ice.
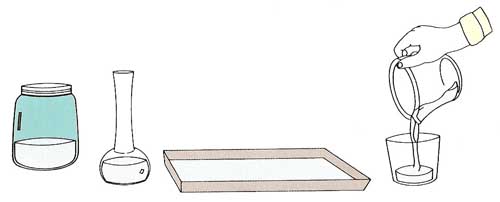
Figure 3. Evaporation experiment.
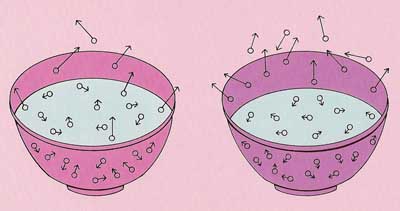
Figure 4. Evaporation at different temperatures.

Figure 5. Sweating helps to cool us down by evaporation.
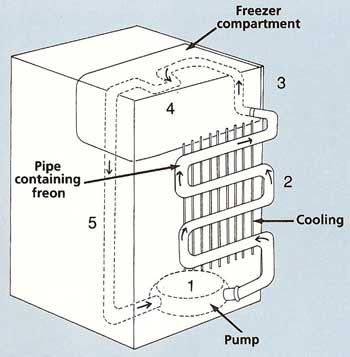
Figure 6. Inside a freezer.
We think of air as being a gas. But if air is cooled down enough, the oxygen and nitrogen in it turn to liquids and eventually to solids. In the same way, we think of iron as being a solid. But if iron is heated to 2,802°F (1,524°C) it becomes a liquid, while at 5,252°F (2,871°C) it becomes a gas.
 |
All substances can exist in these three different states: solid, liquid, or gas. The process of going from one form to another is called change of state.

Shake, Rattle, and RollYou will need:
What to do: Paint about 20 of the peas red and allow the paint to dry. Half fill the jar with peas, including the painted ones (see Figure 1). The peas represent the molecules of a substance. The red paint makes it easier for you to follow what happens to one or more individual peas during the course of the experiment. Shake the jar very gently. Keep you eyes on one of the red-painted peas. It will vibrate without changing its position, just like a molecule in a solid does. Now shake the jar slightly harder. Watch what happens. Shake the jar harder still, in different directions. What happens to the pea-molecules? |
Ice to Steam
Water is one of the commonest and most important chemicals on Earth. It is unusual, though, in that it occurs as a solid, a liquid, and a gas in our everyday lives.
The molecules in solid water, or ice, are fixed together in a kind of framework (see Figure 2). Although they can vibrate, they cannot move around freely. If a block of ice is taken out of a freezer, however, it starts to gain heat energy. This energy makes the ice molecules vibrate faster. Eventually, the molecules gain enough energy to break out of their framework and move around freely. At this point, the ice begins to turn to water. In other words, it melts.
If more heat is given to the water, say by warming it in a pan, the water molecules move even faster. Given enough heat, they will move so quickly that they escape from the surface of the water altogether. The water boils, turning into gas known as water vapor.
Compare how water turns from solid to a liquid to a gas with your observations of peas in the "Shake, Rattle, and Roll" experiment.

Melting IceYou will need:
What to do: Place the ice cubes in a plastic bowl in a warm room. Put the thermometer carefully in the bowl so that it is surrounded by ice. Record the temperature and start the stopwatch (see Figure 2). Take further readings of the temperature every 5 minutes. After each reading, gently stir the contents of the bowl. Record the time at which the last of the ice disappears. Continue to record the temperature of the water every 5 minutes until it no longer rises. Plot a graph of time against temperature. Draw a line to show when the last of the ice melted. Can you draw any conclusions from your graph? |
Changes of State
 |
While a substance is changing state, its temperature remains the same. For example, the temperature of boiling water is 212°F (100°C). Supplying more heat to a pan of boiling water does not raise its temperature; it simply makes the water boil faster.
In the same way, ice melts at 32°F (0°C). The temperature of a melting ice-water mixture remains the same – 32°F (0°C) – until all the ice has melted.
The opposite of melting is freezing. Both happen at the same temperature. For example, freezing water stays at 32°F (0°C) until it has all turned to ice. Only then can it be cooled to lower temperatures.
The opposite of boiling is CONDENSATION. This happens when molecules of gas start to stick together and form droplets of liquid. When water in a pan boils, for example, it turns to water vapour, which is an invisible gas. But almost immediately the water vapour condenses into tiny droplets in the air, which we call steam.

Going, Going, GoneYou will need:
What to do: Measure out 8 ounces of water and pour it carefully into one of the containers. Repeat this with the other containers. Place all of the containers in the same place in a warm room (see Figure 3). Leave them for one week. Pour the contents of each container back into the measuring cup and record the quantity of water for each . What do you notice? Has the amount of water gone down? If so, in which containers has it gone down the most? Try to explain your results.
Repeat the experiment using two containers of the same size and shape. Pour the same amount of water into each. Put one of the containers in a cool place, such as a cupboard. Put the other in a warm place, such as a sunny window ledge. After one week, measure the amount of water remaining. What can you conclude from this? |
Evaporation
A liquid will turn to gas if it heated to its boiling point. Bit a liquid will also slowly turn to gas at lower temperatures. This is called EVAPORATION (see Figure 4).
The reason liquids evaporate is that some of the molecules inside them are moving faster than others. The fastest of all have enough energy to escape from the liquid, even though the temperature may be well below the boiling point.
Since a molecule has to break free of a liquid's surface in order to escape, the rate at which a liquid evaporates depends on how much of its surface is exposed to air. Water that is spread out as a shallow puddle, for example, will evaporate much faster than the same amount of water contained in a bottle. The rate of evaporation also increases as the temperature rises.

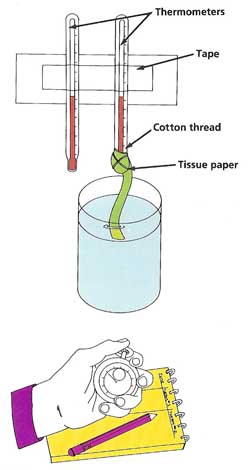
Cool It!You will need:
What to do:
Wrap the top of the strip of tissue paper around the bulb of one of the thermometers. Tie it in place with some thread. Tape the thermometers side by side against a wall or other support. After filling the glass with water, leave it for an hour to come to room temperature. Note the readings on the two thermometers (they should be the same). Dip the free end of the tissue paper into the water, so that the water soaks up to the bulb of the thermometer. Start the stopwatch. Take the readings of both thermometers at 1-minute intervals. Plot a graph of the results. What are your findings? Can you think of an explanation?
Taking it further: Remove the tissue strip from the thermometer. Wait until both thermometers show the same temperature. Attach the tissue strip again and set up the experiment as before. Start the stopwatch. Turn on the fan and point it at the thermometers from a distance of a few feet. Repeat the rest of the experiment as before. Compare your results. Is there any difference? Again, try to explain your observations.
Warning: Never touch an electric appliance, such as the fan in this experiment, if your hands are wet. You may receive a dangerous shock. |
Cooling by Evaporation
The temperature of a substance depends on the average energy of all its molecules. If the fastest molecules get away, then the average energy of the molecules that are left will be lower – and the substance will be cooler. For this reason, evaporation causes a liquid to cool down (see Figure 5).
When we are hot, we sweat. As the fastest water molecules in the sweat escape, they lower the temperature of the remaining liquid. This, in turn, helps to cool our skin and the blood that flows beneath it.
If the air over an evaporating liquid is still, a tiny cloud of water vapor collects near the liquid's surface. This makes it harder for other molecules to escape. However, if the air is moving, the cloud of water vapor is blown away. This enables other molecules in the liquid to escape more easily so that the rate of evaporation – and the rate of cooling – are increased. To see this idea at work, lick the back of your hand; then blow across it. Blow gently at first and then harder. What do you feel?
Inside Freezers
Cooling by evaporation gives us a way to make ice and to preserve food by freezing it. Look behind a refrigerator and you will see some twisting pipes behind a line of metal "fins." The pipes contain a substance called Freon, which changes very easily from liquid to vapor and back again (see Figure 6).
The Freon starts its journey around the refrigerator by being squeezed out of a pump [1] as a vapor at high pressure. It passes up through the pipes at the back of the refrigerator [2], losing heat as it turns into a liquid. The metal fins are designed to get rid of this heat, which is why they feel warm. Next, the Freon is squirted through a narrow opening [3] into a wider pipe that passes round the inside of the freezer compartment [4] and the rest of the refrigerator [5]. This causes the Freon to evaporate and cool its surroundings. Finally, the Freon returns to the pump to begin its journey all over again.
 |