Boyle's law

Figure 1. Boyle's law implies that if a quantity of an ideal gas is to be compressed at constant temperature so that its volume is reduced by one half (from v to v/2) its pressure must be doubled (from p/2 to p).
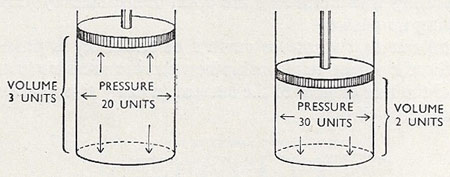
Figure 2. The inverse relationship between pressure and volume is evident in this diagram.
Boyle's law states that for a given mass of gas held at a constant temperature, pressure (p) and volume (v) are inversely proportional. That is,
pv = constant.
Boyle's law is strictly true only for an ideal gas. It is named after Robert Boyle who reported it in 1662, but was actually discovered by Boyle's assistant R. Townely.
The French physicist, plant physiologist, and priest Edmé Mariotte (1620–1684) discovered the law independently in 1676, and in France it is known as Mariotte's law. (Mariotte, a founder of the Academy of Sciences in Paris) also concluded that plants synthesize materials by chemical processes, a theory verified after his death.


