butane
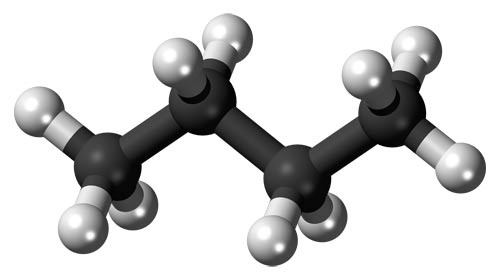
Ball-and-stick model of a butane molecule.
Butane (C4H10) is a colorless, flammable gas, the fourth member of the alkane series of hydrocarbons. It has two isomers: n-butane is obtained from natural gas; isobutane (2-methylpropane) is made by the cracking of petroleum. Butane can be liquefied under pressure at normal temperatures and is used in bottled gas. It is also used in the manufacture of 1,3-butadiene and high-octane gasoline. Boiling point: (n-butane) –0.3°C (31.5°F) and (isobutane) –10.3°C (13.5°F).


