hydrocarbon
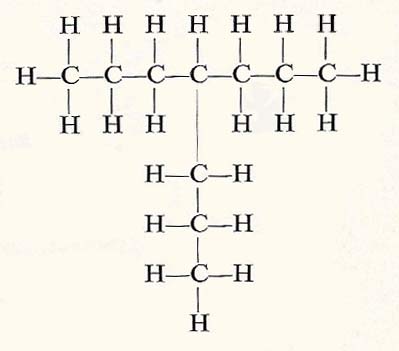
A hydrocarbon with a branching chain. The substance shown here has properties that are slightly different from those of the single-chain substqnce with the same number of carbon and hydrogen atoms.
A hydrocarbon is a general term for any organic compound that contains only carbon and hydrogen. Like organic compounds in general, which are derived formally from hydrocarbons by adding functional groups, they can be divided into aliphatic, alicyclic, and aromatic hydrocarbons. Aliphatic hydrocarbons are further subdivided into alkanes, alkenes, and alkynes.
Some hydrocarbons, especially terpenes, occur in plant oils, and solid, high-molecular-weight hydrocarbons occur in bitumen, but by far the largest sources of all sorts of hydrocarbons are petroleum, natural gas, and coal gas. They are used as fuels, for lubrication, and as starting materials for a wide variety of industrial syntheses.
Fluorocarbon
A fluorocarbon is a hydrocarbon in which hydrogen atoms are replaced (wholly or in part) by fluorine. Because of the stability of the carbon-fluorine bond, they are inert and heat-resistant. Thus they can be used in artificial joints in the body, and where hydrocarbons would be decomposed by heat, such as in spacecraft heat shields, the coating of nonstick pans, or as lubricants (see lubrication). Liquid fluorocarbons are used as refrigerants.


