clathrate
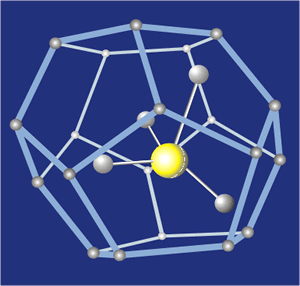
Methane hydrate.
A clathrate is a solid material in which small molecules of one substance (called the guest
molecules) are trapped and enclosed in spaces in the crystal lattice of
another substance (called the host molecules). The word comes from the Latin clathratus meaning "furnished with a lattice." Clathrates are sometimes
called cage compounds or enclosure compounds.
Methane hydrate, for example, is a clathrate in which methane molecules, typically released by bacterial decay, become caged within the
open-structured lattice of water ice. It is
found commonly in permafrost regions
in Siberia and North America and is widespread on the seafloor in the vicinity
of continents. The release of methane from these reserves, as the ice melts,
has been cited as a potentially significant factor in globally warming.
Methane hydrate is also among the clathrate hydrates that have been identified
in the ice of cometary nuclei. Quinol
is another substance that forms a variety of clathrates with substances
such as xenon and sulfur
dioxide.
Researchers have begun to investigate silicon and germanium clathrates for possible semiconducting and superconducting properties.


