dipole
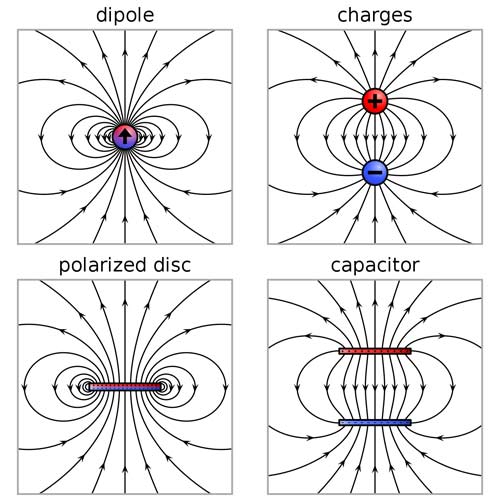
The electric field due to a point dipole (upper left), a physical dipole of electric charges (upper right), a thin polarized sheet (lower left) or a plate capacitor (lower right). All generate the same field profile when the arrangement is infinitesimally small.
A dipole is a separation of charges, as in the case of some molecules. In a covalent bond between the two atoms the electron pair is not necessarily equally shared between them. In hydrogen chloride (HCl), the electrons are attracted toward the more electronegative chlorine atom, giving it a partial negative charge and leaving an equal positive charge on the hydrogen atom. Such dipoles contribute to the the chemical properties of molecules.
Magnetic substances have dipoles consisting of north and south poles; they may be ordered or disordered to give strong or weak magnetic properties.


