electronegativity
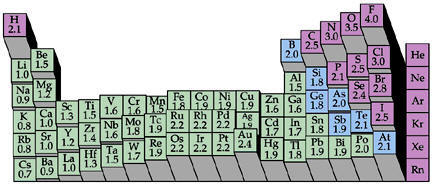
Electronegativity is a measure of the tendency of an atom in a stable molecule to attract electrons within bonds. Elements with low electronegativities (metals and metalloids) tend to lose electrons and become cations. Elements with high electronegativities (nonmetals) tend to gain electrons and become anions. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen resulting in a polar molecule with negative charge on the chlorine atom.
In general, electronegativity values increase from left to right across each row of the periodic table and decrease down each group.
Various schemes have been devised to assign values for the electronegativity of an element. Pauling electronegatives, which are commonly used, are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has a value of 4.


