chlorine

Figure 1. Chlorine gas in a bottle showing its distinctive coloration.

Figure 2. The industrial preparation of chlorine involves the
electrolysis of brine, the reaction being 2NaCl +2H2O →
Cl2 + 2NaOH + H2. The diaphragm cell shown here
is one of the methods used.
1. Brine (salt solution) inlet. 2. Chlorine produced at the graphite
anodes bubbles up through the brine reservoir. 3. Graphite anode. 4.
Pipeline connected to the cells collects this chlorine; pipeline is
rubber-lined to prevent corrosion. 5. Hydrogen gas and sodium hydroxide
produced inside the iron screen cathode pockets. 6. Sodium hydroxide
outlet through which the sodium hydroxide produced inside the pockets
flows. 7. Hydrogen gas led off from the cell. 8. Concrete cell (Hooker
diaphragm type).
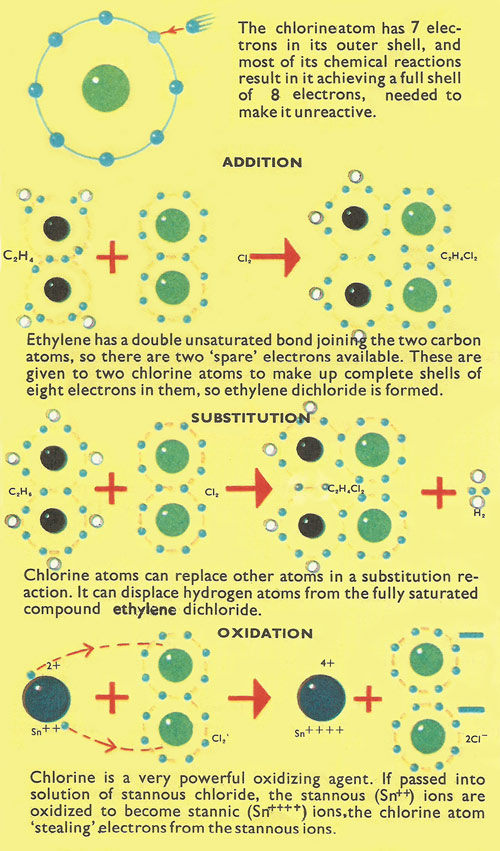
Figure 3. Types of reactions involving chlorine.
Chlorine (Cl) is a poisonous, greenish-yellow, gaseous element, with a choking smell (Figure 1). Chlorine is one of the halogens. It occurs widely in nature as sodium chloride in seawater and as halite (NaCl), carnalite (KCl.MgCl2.6H2O), and sylvite (KCl). It is washed out of rocks and carried down to the seas by rivers, where it forms more than 3% of the ocean’s weight. It is found in some parts of the world in salt beds, (old dried up seas) which are sometimes more than 60 meters in depth. From these, sodium chloride is quarried in the form of rock salt. Chlorine gas, on its own, is never found in nature as it is far too reactive.
Chlorine is more than twice as dense as air and is quite soluble in water. When thoroughly dried and purified, it is not very reactive but in the presence of small traces of water, (always there when the gas is prepared in the laboratory or in industry) it becomes very active.
Chlorine does not burn but is a very reactive substance, combining directly with many elements, both metals and non-metals (e.g., sodium and phosphorus) and compounds. In some reactions it acts a strong oxidizing agent by removing hydrogen from compounds. The hydrogen combines with the the chlorine to yield hydrogen chloride.
Apart from its appearance and pungent odor a test for chlorine gas is to see whether moist red litmus paper is bleached by the gas. The action of chlorine as a bleaching agent is one example of its oxidizing properties. The gas itself does not act as a bleach because the dry gas does not affect a piece of dry litmus paper. The bleaching action is caused by formation of hypochlorous acid (HOCl) when chlorine is dissolved in water:
Cl2 + H2O → HCl + HOCl
When combined with oxygen, the chlorine atom forms a hypochlorite radical (OCl) that seems to possess bleaching properties. Thus, the formula of bleaching powder is often represented by Ca(OCl)2, although the true structure is rather more complicated than this.
Chlorine reacts with most organic compounds, replacing hydrogen atoms (see alkyl halides) and adding to double and triple bonds (see section "Reactions of chlorine" below).
Chlorine was discovered by Carl Scheele in Uppsala, Sweden, in 1774. Its name comes from the Greek chloros, meaning "pale green".
| atomic number | 17 |
| atomic mass | 35.453 |
| electronic configuration | 1s22s22p63s23p5 |
| first ionization energy | 1,251 kJ/mol |
| electronegativity | 3.2 |
| atomic radius | 99 pm |
| ionic radius | 181 pm |
| density | 3.214 g/dm3 |
| melting point | -101.5°C (171.6 K) |
| boiling point | -34.04°C (239.1 K) |
Preparation of chlorine
In the laboratory, chlorine may be prepared by the oxidation of hydrochloric acid – hydrogen ions are oxidized to water thus releasing the free element chlorine. The usual way of bringing about the reduction is to heat manganese dioxide with concentrated hydrochloric acid.
2MnO2+ 8HCl → 2MnCl2 + 2Cl2 + 4H2O
Chlorine is also released by the action of hydrochloric acid on either potassium permanganate or bleaching powder. Although the gas is soluble in water, it may be collected over a strong solution of sodium chloride.
Chlorine is also obtained by the electrolysis of sodium chloride solution using carbon electrodes. Chlorine is liberated at the anode (positive electrode) while sodium is set free at the cathode (negative electrode). However, sodium is so reactive that it reacts with the water – hydrogen is liberated at the same time as sodium hydroxide is formed.
On an industrial scale, although chlorine was for many years produced by chemical means, electrical processes are now used. In the Kellner-Solvay process a layer of mercury flows slowly over the bottom of a cell. The mercury forms a cathode and graphite anodes are suspended, in a brine solution, above the mercury. The sodium ions (Na+) can travel to the mercury and form an amalgam with it. The sodium-mercury amalgam then flows into a water-filled vessel where the sodium attacks the water and forms soluble sodium hydroxide, whilst the pure mercury flows on. The chloride ions (Cl-) are passed to the cathode where they lose their charge and form chlorine gas.
This method has now been largely superceded by diaphragm cell electrolysis (Figure 2) and membrane cell electrolysis.
Uses
Chlorine is used in large quantities as a bleach, as a disinfectant for drinking water and swimming pools, and in the manufacture of plastics, solvents, and other compounds.
Reactions
A large range of chemical substances combines very readily with it – for example sulfur, arsenic and phosphorus all burn vigorously in chlorine, and in the presence of strong light, hydrogen and chlorine combine explosively. Chlorine gas also combines with zinc and iron to form chlorides when heat is applied, but will attack other metals only in the presence of traces of moisture. It liquefies at about –34°C to a dark green liquid.
Addition and substitution reactions
Two important properties of chlorine are the ability to take part in addition and substitution reactions (Fig 3). In the addition reaction an unsaturated compound – one that can accept additional atoms to use all the available valence bonds – takes in the chlorine atom. Chlorine, like all halogens, can be accepted in this way because it needs an additional electron to make up a full quota of eight electrons in its outer electron shell, and the atoms in the unsaturated compounds can provide them.
Addition reactions take place with both inorganic and organic unsaturated compounds. For example, carbon and oxygen are held together in carbon monoxide by a 'double' unsaturated bond, which can be broken into by chlorine to form the saturated compound, phosgene:
CO + Cl2 → COCl2
The unsaturated organic compound ethylene is saturated by chlorine to form ethylene dichloride:
C2H4 + Cl2 → C2H2Cl2
In substitution reactions, chlorine atoms show the ability to push out other atoms and take their places in saturated compounds. For example, it displaces hydrogen from both ammonia and methane to form nitrogen trichloride and carbon tetrachloride:
These reactions normally take place in a number of stages and in fact the partly substituted chloride of methane, CHCl3, is the well-known compound, chloroform.
Chlorine as an oxidizing agent
Another important chemical property of chlorine is that it can act as a powerful oxidizing agent. It should be remembered that a proper definition of oxidation is not necessarily concerned with oxygen but with the loss of electrons from the atom. For example, when chlorine is passed into stannous chloride solution the stannous ions are oxidized to become stannic ions:
SnCl2 + Cl2 → SnCl4 or
Sn2+ + Cl2 → Sn4+ + 2Cl-.
Once again, this is an example of chlorine atoms, short of a single electron to make up the 'magic number' of eight in the outer shell, 'stealing' a single electron from another atom.
Compounds of chlorine
Chlorides
Chlorine, in combination with other elements, gives rise to a series of compounds called chlorides. The gas hydrogen chloride (HCI) dissolves in water to form hydrochloric acid, a most important chemical reagent. The family of metallic chlorides is formed by the action of hydrochloric acid on the metals themselves. For example, when the acid acts on metallic zinc, zinc chloride is formed:
Zn + 2HCl → H2 + ZnCl2
Chlorides are also formed by the action of hydrochloric acid on metallic oxides. Then, water is liberated instead of hydrogen.
Chlorides, the commonest chlorine compounds, are typical halides except for carbon tetrachloride (see carbon), which is inert. Most chlorides are soluble in water, except mercury (I) (mercurous) and silver chlorides.
Oxides and oxyanions of chlorine
Other chlorine compounds include a series of oxides, unstable and highly oxidizing: dichloric monoxide Cl2O, chlorine dioxide ClO2, chlorine trioxide ClO3, and dichloric heptoxide Cl2O7. There is also a series of oxyanions – hypochlorites, chlorites, chlorates (salts of chloric acid, containing the ClO3- ion), and perchlorates – and corresponding oxy-acids: hypochlorous acid (HOCl), chlorous acid (HClO2), chloric acid (HClO3), and perchloric acid (HClO4), all of which are powerful oxidizing agents. Calcium hypochlorite (see bleaching powder and sodium chlorite are used as bleaches; chlorates are used as weedkillers and to make matches and fireworks; perchlorates are used as explosives and rocket fuels.
Chlorine and the other halogens
Chlorine is the second member of the seventh group of the periodic table – the halogens. All members of this group have seven electrons in the outer shell and they have many properties in common. In fact, the halogen family serves as an excellent demonstration of the way the periodic classification works. All of the members – fluorine, chlorine, bromine, and iodine are oxidizing agents and take part in substitution and addition reactions. As the members of the family become heavier and heavier, however, the reactivity is not so great. Fluorine is thus a stronger oxidizing agent then chlorine, for example, and chlorine a stronger oxidizing agent than bromine.
The physical properties are likewise similar, with the boiling and melting points increasing with an increase of the atomic weight.


