formic acid
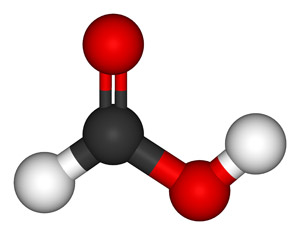
Formic acid (HCOOH), also known as methanoic acid (its systematic name), is a colorless, corrosive, pungent liquid, which is the simplest and strongest carboxylic acid. Formic acid occurs in the stings of ants and nettles. Formic acid is made (via sodium formate) by heating sodium hydroxide with carbon monoxide under pressure. It is used in tanning, as a latex coagulant, in electroplating, and to reduce dyes. The aldehyde derivative of formic acid is methanal.
| relative molecular mass | 46.0 |
| density relative to water | 1.22 (at -20°C) |
| melting point | 8.3°C (46.9°F) |
| boiling point | 100.8°C (213.4°F) |


