ground state
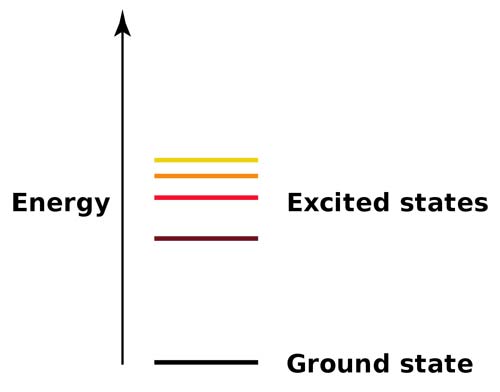
Energy levels for an electron in an atom: ground state and excited states. After absorbing energy, an electron may jump from the ground state to a higher-energy excited state.
The ground state is the condition of an atom, ion, or molecule, when all of its electrons are in their lowest possible energy levels, i.e., not excited. When an atom is in its ground state, its electrons fill the lowest energy orbitals completely before they begin to occupy higher energy orbitals, and they fill subshells in accordance with Hund's rule (usually!)
When an electron temporarily occupies an energy state greater than its ground state, it is in an excited state. An electron can become excited if it is given extra energy, such as if it absorbs a photon, or packet, of light, or collides with a nearby atom or particle. Electrons do not stay in excited states for very long - they soon return to their ground states, emitting a photon with the same energy as the one that was absorbed.
In the case of an atomic nucleus, the ground state is the lowest, most stable energy state of a nucleus.


