heavy water
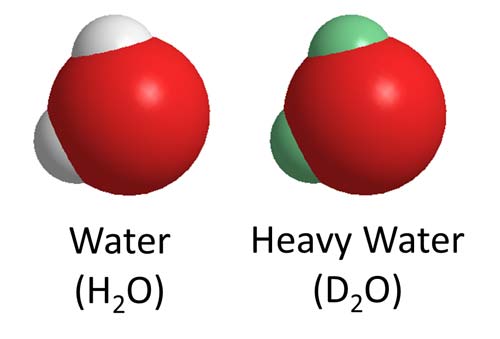
Heavy water differs from regular water only in that the H atoms (white) in water are replaced by deuterium (green) in heavy water. The oxygen atoms are shown in red.
Heavy water (D2O), deuterium oxide, is water in which ordinary hydrogen atoms, 1H, are replaced by atoms of the heavier isotope of hydrogen known as deuterium, 2H (represented by the symbol D). Heavy water or deuterium oxide, D2O, is a colorless liquid, which forms hexagonal crystals on freezing.
| density relative to water | 1.105 |
| melting point | 3.8°C |
| boiling point | 101.4°C |
In ordinary water there are 6,500 ordinary H2O water molecules to every molecule of heavy water. D2O can be separated by fractional distillation or electrolysis.
Heavy water is used in the nuclear industry as a moderator because of its ability to reduce the energies of fast neutrons to thermal energies and also because its absorption cross-section is lower than that of hydrogen so that it does not significantly reduce the neutron flux.


