hydroxide
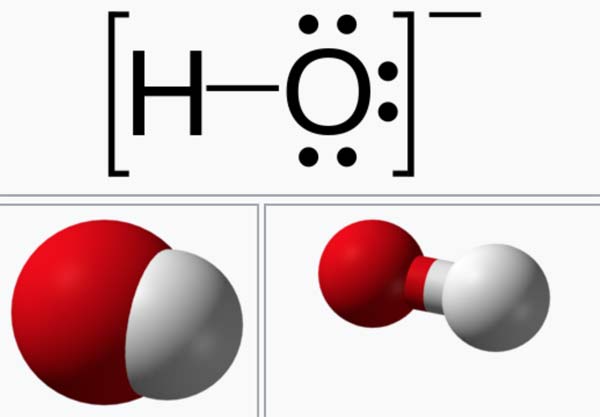
A hydroxide is a compound that contains the OH group, or the ion OH-. Hydroxides of metals are generally bases, and, if soluble, ionize to produce alkaline solutions (see alkali) containing hydroxide ions. Nonmetals form acid hydroxides, or oxyacids, which dissolve to produce hydrogen ions. Some metal hydroxides, such as zinc hydroxide, are amphoteric, that is, both basic and acidic.
Hydroxides are formed by hydration of the oxide, or, if insoluble, by precipitation with an alkali. The OH– ion acts as a ligand, forming hydroxo complexes. Organic compounds containing the OH group (see hydroxyl) are alcohol, phenols, and carboxylic acids.


