phenol
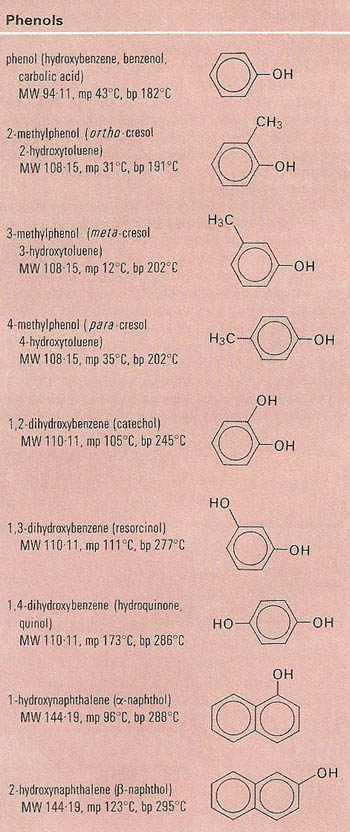
The term 'phenol' may refer to either a specific compound or a family of compounds. Phenols are aromatic compounds in which a hydroxide group is directly bonded to an aromatic ring system. They are very weak acids, and, like alcohols, form ethers and esters. They are very liable to undergo electrophilic substitution (see electrophile), and hence condense with methanal (formaldehyde) to form resins. The main phenols are phenol itself, cresol, resorcinol, pyrogallol (see gallic acid), and picric acid.
Phenol itself (C6H5OH), also known as carbolic acid, is a white, hygroscopic crystalline solid, insoluble from coal tar, but made by acid hydrolysis of cumene hydroperoxide, or by fusion of sodium benzenesulfonate (see sulfonic acid) with sodium hydroxide. Formerly used as an antiseptic, phenol has more latterly been used to make Bakelite and other resins, plastics, dyes, detergents, and drugs. Molecular weight 94.1, melting point 43°C, boiling point 182°C.
Cresol
Cresol, also known methylphenol (CH3C6H4OH), is a member of the phenols. There are three isomers, synthesized from toluene. Distillation of creosote or petroleum yields a mixture of cresols and xylenols ("cresylic acid") used as a disinfectant and in the manufacture of resins and tricresyl phosphate (used to make gasoline additives and plasticizers).
Picric acid
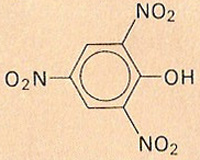 |
Picric acid, also known as 2,4,6-trinitrophenol, C6H2OH(NO2)3, is a yellow crystalline solid, made by nitration of phenol, or its derivatives. Picric acid is a moderately strong acid, it has been used as a dye, as an antiseptic and astringent for treating burns, and as a high explosive. Molecular weight 229.1, melting point 122°C.
Resorcinol
Resorcinol, m-C6H4(OH)2, is one of the dihydric phenols, a colorless crystalline solid made by sulfonation (see sulfonic acid) of benzene followed by fusion with sodium hydroxide. Resorcinol is used to make adhesives, methanal (formaldehyde) resins, dyes, explosives, photographic developers, and as an antiseptic. Molecular weight 110.1, melting point 111°C, boiling point 281°C.


