nitration
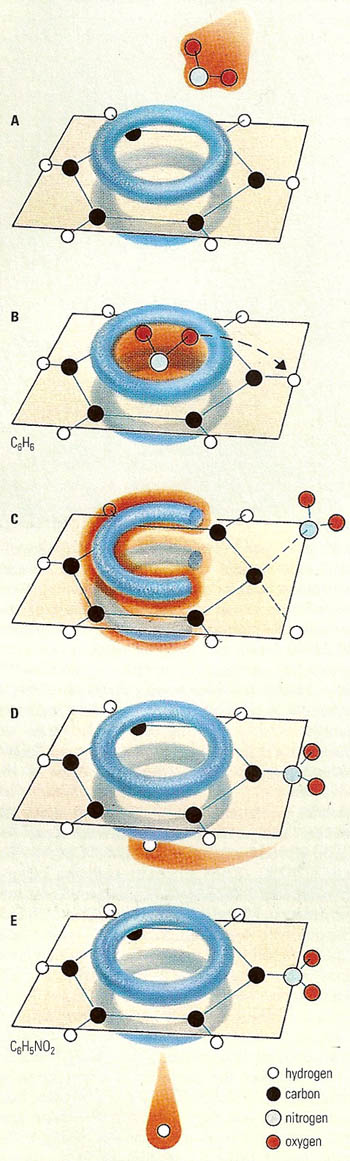
Nitration of benzene.
Nitration is an important process in organic chemistry, in which a nitro group (–NO2) is introduced into a compound. Aromatic compounds are nitrated with a mixture of concentrated sulfuric acid and nitric acid, which contains the electrophile; the production of TNT and nitrobenzene are important cases. "Nitration" is a term also used to describe the formation of nitrate esters including nitrocellulose and nitroglycerin.
Illustrated to the right is the nitration of benzene, which produces a reaction that goes through several stages. Initially (A), the entering group approaches and associates weakly with the benzene ring (B). Then, rearrangement produces an unstable high-energy intermediate (C), which breaks down to a complex (D) in which the leaving group is weakly associated with the ring. It ends with the departure of the leaving group (E).


