nitrobenzene
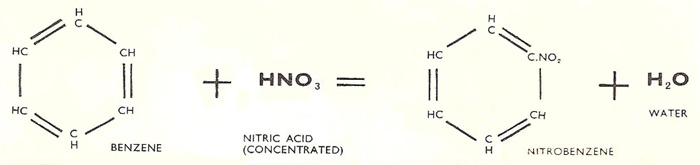
The double decomposition reactionp in which nitrobenzene is formed.
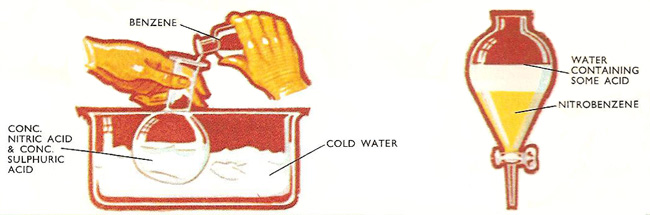
Preparation of nitrobenzene shown diagramatically.
Nitrobenzene (C6H5NO2) is a colorless, highly refractive liquid with a characteristic smell. It is manufactured by reacting benzene with a mixture of concentrated nitric acid and concentrated sulfuric acid. The benzene and nitric acid undergo a double decomposition reaction which also produces water:
The sulfuric acid acts as a dehydrating agent. Since a lot of heat is generated by the reaction and since an unwanted substance, dinitrobenzene, is formed if the temperature of the reaction mixture rises above 50°C, the reaction vessel must be cooled. When the reaction is complete, the mixed products are poured into cold water. Nitrobenzene is more dense than water and is insoluble in it, so forms a separate layer beneath the water. In this way nitrobenzene is separated and can be further purified by fractional distaillation.
Most of the nitrobenzene produced is used to manufacture aniline, which is obtained by reduction. A considerable proportion is used as a raw material in the dyestuffs industry (see dye), as either as nitrobenzene itself, or as aniline.
Further nitration gives 3-dinitrobenzene; sulfonation gives 3-nitrobenzene sulfonic acid. Reduction gives first azoxybenzene, then azobenzene and aniline, depending upon the conditions.
Melting point 6°C, boiling point 211°C.


