aniline
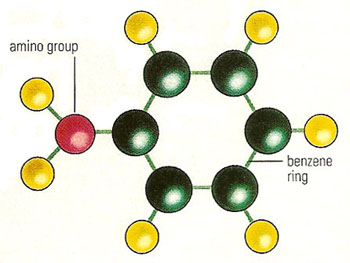
Figure 1. Aniline molecule.

Figure 2. Natural plant dyes.

Figure 3. p-aminoazobenzene and phenylazo-2-napthol.
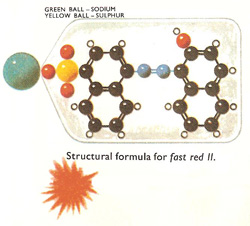
Figure 4. Fast red II.
Aniline (C6H5NH2), also known as phenylamine or aminobenzene, is a colorless, oily, highly toxic liquid; its vapor has a distinctive odor and is poisonous. It is made by the reduction of nitrobenzene (see below) or by reaction of chlorobenzene with ammonia. Structurally, aniline consists of a planer benzene ring, with one amino group giving the molecule basic properties (Figure 1); it is a primary aromatic amine.
Aniline is an important starting point for making organic compounds, including some drugs, synthetic dyes, resins, and explosives. It is used in the manufacture of rubber tires, where it acts as an accelerator in the process of vulcanization by catalyzing the chemical reactions involved – shortening the heating time needed. It was also used as a propellant for some early rockets, such as the American Corporal. However, because of its toxicity, it is no longer used as a rocket fuel.
| relative density | 1.02 |
| melting point | -6.2°C (20.8°F) |
| boiling point | 184.1°C (363.4°F) |
Preparation
An amino group cannot be introduced into the benzene ring by a single chemical reaction. Instead, nitrobenzene (C6H5NO2) is formed first and is then reduced in a second reaction which yields aniline.
Nitrobenzene is made, both in the laboratory and commercially, by slowly and carefully adding benzene to a mixture of concentrated nitric acid and concentrated sulfuric acid. Aniline may then be obtained from the nitrobenzene by reducing it using nascent hydrogen (i.e., hydrogen that is actually prepared in the presence of the nitrobenzene).
Properties
Aniline may be regarded as a derivative of ammonia – one of the hydrogen atoms in the ammonia molecules has been replaced by a phenyl (C6H5–) group. It is not surprising, therefore, that aniline behaves as a base – aniline forms salts with acids, for example, with hydrochloric acid it forms aniline hydrochloride:
C6H5NH2 + HCl → C6H5NH3Cl
Aniline is to ammonia as aniline hydrochloride is to ammonium chloride.
Aniline is readily oxidized. Various oxidizing agents yield different products. Thus aniline black is obtained by the action of potassium dichromate in acid solution. It was, in fact, this reaction which led William Perkin to his investigation of the coal-tar dyes. Aniline even undergoes oxidation when exposed to the air and as a result it takes on a dirty red-brown color.
In common with the aliphatic amines (i.e., amines of the open chain hydrocarbons), nitrogen is liberated when nitrous acid is added to aniline. Phenol and water are also produced in this reaction:
C6H5NH2 + HNO2 → C6H5OH + N2 +H2O
However, if the temperature of the solution is kept below 10°C and if it is acidified with hydrochloric acid a diazonium salt is obtained:
C6H5NH3Cl + HNO3 → C6H5N2Cl + 2H2O
The diazonium compounds contain the azo (–N = N–) group and are the intermediate in the preparation of the azo group of dyes. As the diazonium salts are difficult to isolate and are explosive in the solid state they are usually kept in aqueous solutions.
History of dyes
It is little more than a century and a half since the first synthetic dye was prepared by William Perkin. Before 1857 almost all organic dyes and pigments were obtained from vegetable or animal sources (Figure 2). The yellow dye saffron, for example, was obtained from the yellow stigmas of a blue crocus, Crocus sativus, which was grown in countries around the Mediterranean. A shell-fish, Murex brandaris, was the source of the expensive dye, Tyrian purple.
The amount of dye which can be obtained from one plant or animal is quite small, and, in consequence, the process of extraction tends to be costly. The production of Tyrian purple is, perhaps, exceptional, but it is recorded that 8,500 specimens of the shell-fish were used to make 1 gram of dye. In contrast, the chemical reactions used in making synthetic dyes have very good yields, and the products do not generally require much further treatment. Synthetic dyes are, therefore, cheaper and, furthermore, can be tailor-made to suit particular circumstances.
Although the first dye, mauveine, which Perkin made, was not itself an azo compound (i.e., a substance containing an –N = N–, or azo, group) it was obtained using the same raw material, aniline, use in making azo dyes. Aniline, the simplest of the aromatic amines is made from benzene, and since benzene is a constituent of coal tar, this whole family of dyes is known as the coal-tar dyes.
Not only is benzene found in coal-tar, large quantities are also obtained on a commercial scale from oil. In both instances the benzene is mixed with other substances, so before it is fit for use, it must be purified. Benzene from coal-tar is more difficult to refine since it is contaminated with phenol, pyridine, and thiophene, and also contains other aromatic hydrocarbons (xylenes and toluene).
Aniline and the azo dyes
Benzene diazonium chloride (and the diazonium compounds formed from other aromatic amines) readily react with phenol and aromatic amines to form a whole range of brightly colored dyes. Thus a yellow dye p-amino azobenzene, is obtained when one aniline molecule couples with a molecule of benzene diazonium chloride (Fig 3). However, if 2-napththol is used instead of aniline, a red dye, phenylazo-2-napthol is obtained. In practice the more satisfactory azo-dyes consist of larger molecules.
It is the azo group itself which is responsible for imparting a color to the compound. The azo group is, therefore, known as a chromophore. By varying the groups that are coupled to the azo group new dyes with different and better colors can be produced. Any groups, such as amino, hydroxyl, and halides, which modify the dolor of a dye are called auxo-chromes.
Azo-dyes are particularly useful for dying wool, while synthetic dyes of other types are more suited to coloring other fabrics. Whereas some substituent groups impart various colors, others enable the dye to be used with different types of cloth or to serve under different conditions, such as in strong sunlight or in very moist air.


