amine
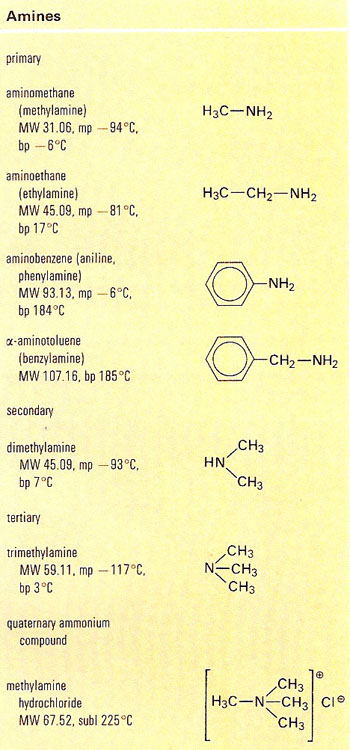
An amine is an organic compound formed by replacing one or more of the hydrogen atoms
in the ammonia molecule (NH3) by alkyl
groups or aryl groups. Primary amines
have general formula RNH2; secondary R2NH; tertiary
R3N. Heterocyclic nitrogen bases (including the alkaloids and pyridine) are tertiary amines.
Amines may be formed by reduction of amides, nitriles, or nitro compounds, or by reaction
of ammonia with organic halides, alcohols,
or sulfonic acids. They are produced
in nature by the putrefaction of organic matter.
Simple amines are pungent liquids which are strong bases and ligands; many occur naturally in decaying organic matter. They give amides with acid derivatives. All amines react with nitrous acid (dilute hydrochloric acid and sodium nitrate) but the product varies according to the number and type of hydrocarbon units attached to the nitrogen atom in the amine group. If the amino group is attached directly to a hydrocarbon ring and the reaction is carried out at 5°C or below, a diazonium salt is formed. Diazonium salts are the starting points in the manufacture of azo dyes. Amines are also used in the manufacture of many drugs and synthetic fibers.


