cyanide
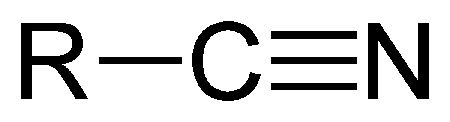
The nitrile group.
A cyanide is an inorganic compound that contains the –CN group. Organic compounds that contain the –CN group group are called nitriles, although they may also be referred to as organic cyanides.
Cyanides are salts or esters of hydrocyanic acid (HCN), a volatile weak acid; both are highly toxic. Sodium cyanide (NaCN) is made by the Castner process: ammonia is passed through a mixture of carbon and fused sodium. Both sodium cyanide and potassium cyanide (KCN) are both deadly poisonous and have a characteristic smell of almonds. The cyanide ion (CN-) is a pseudohalogen, and forms many complexes. Cyanides are used in the extraction of gold and silver, electroplating, and casehardening (a simple method of hardening steel).
Hydrogen cyanide
Hydrogen cyanide (HCN) is a colorless liquid or gas at normal temperatures, which is completely miscible with water, burns in air, smells of bitter almonds, and polymerizes on standing. Hydrogen cyanide forms cyanides (RCN) and isocyanides (RNC). It is used in organic and synthetic fiber synthesis and as a fumigant, and is a very strong poison.
Hydrogen cyanide is central in the prebiotic synthesis of amino acids and purines. The fact that it occurs abundantly in comets has fueled speculation that cometary collisions may have played a significant role in supplying some of the raw materials for prebiotic evolution.
| density relative to water | 0.70 (at 22 °C) |
| melting point | -14 °C |
| boiling point | 26 °C |
Hydrocyanic acid
Hydrocyanic acid, also called prussic acid, is a solution of hydrogen cyanide in water; a deadly poisonous weak acid. It is a liquid with an odor of bitter almonds, sometimes used as a fumigant to destroy rats and other pests of buildings. It also has uses in the chemical industry but is so dangerous to handle that its solid salts potassium and sodium cyanide are preferred, even though these, too, are highly poisonous.
Nitriles
A nitrile is an organic compound containing the –CN group. Nitriles are named for the carboxylic acid to which they can be hydrolyzed. The simplest is acetonitrile (or methyl cyanide), CH2CN. Nitriles are prepared by dehydration of amides or by the reaction of sodium cyanide with alkyl halides or aryl sulfonates.
The aliphatic or alkyl nitriles are important in ascending the homologous series, since they enable an extra carbon atom to be introduced into the molecule. The alkyl nitriles are reduced by hydrogen to give primary amines, and react with Grignard reagents to yield ketones.
Nitriles are important precursors of amino acids. Industrially, nitriles are used in organic synthesis, notably for the manufacture of acrylic fabrics and synthetic rubber.


