amino acids
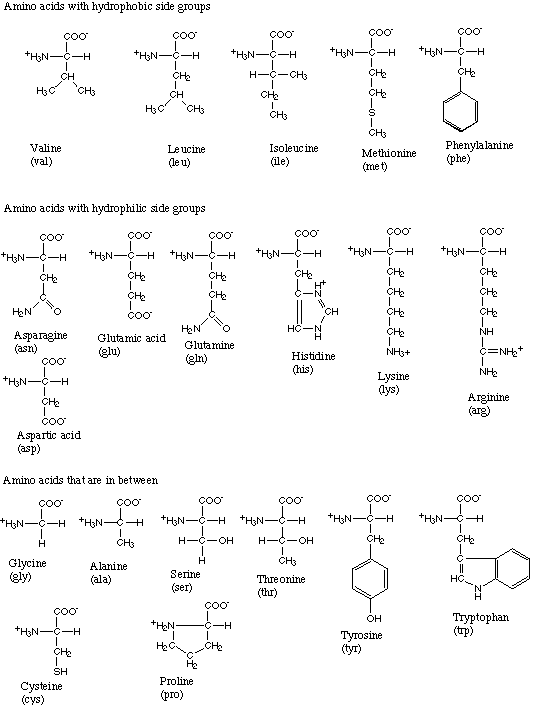
The 20 amino acids found in terrestrial life forms.
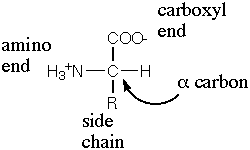
General structure of an amino acid.
Amino acids are the chemical subunits of polypeptides and proteins, and therefore one of the fundamental building blocks of life as we know it. An amino acid consists of a central carbon atom attached to a carboxyl group (–COOH), an amino group (–NH2), a hydrogen atom, and a side group (–R), giving the general formula R-CH-NH2-COOH. Only the side group differs from one amino acid to another; R may be a hydrogen or an organic group and determines the properties of a particular amino acid.
Amino acids link together through a special bond, called a peptide bond, to form peptides, which are short chains, or polypeptides, which are much longer chains. Proteins are made up of various proportions of 20 commonly occurring amino acids (see the table below). The sequence of these amino acids in the protein determines the protein's molecular shape, properties, and biological role.
The amino group
The amino group (–NH2) is a functional group found in all amines and amino acids. It consists of one atom of nitrogen attached by covalent bonds two atoms of hydrogen, leaving a lone valence electron on the nitrogen which is available for bonding to another atom.
Essential amino acid
Plants and many microorganisms can synthesize amino acids from simple inorganic compounds, but animals must obtain the amino acids they need in their diet. So-called essential amino acids are those that an organism, such as a human being, cannot synthesize and therefore has to be ingested as part of the diet. Of the 20 amino acids used in the human body, eight of them are "essential" in adults. They are lysine, threonine, methionine, isoleucine, leucine, valine, phenylalanine, and tryptophan. Two others, arginine and histidine, are essential only in children.
Amino acids and the origin of life
The manufacture of amino acids represents one of the earliest stages in prebiotic molecular evolution and, it turns out, one of the easiest to accomplish in the laboratory. Pioneering work in this field was carried out by Alfonso Herrara in his Laboratory of Plasmogeny in Mexico City, between 1924 and 1942, and by Stanley Miller and Harold Urey in their famous experiment of 1953.
Although a good deal of amino acid synthesis must have taken place in situ on the young Earth, evidence is growing that a contribution came from "seeding" as a result of cometary and asteroidal collisions. Such impregnation of virgin worlds with ready-made organic matter from space, during the early bombardment phase of planetary systems, may occur commonly throughout the Universe and serve further to stimulate prebiotic chemistry where conditions allow. The discovery of amino acids in space provides powerful support for this idea. However, it is unclear how important this cosmic seeding is compared with planet-grown prebiotic material and whether it could actually prove decisive in the development of life.
Left- and right-handed amino acids
Twenty different amino acids are found in the proteins of terrestrial life-forms. All of these, with the exception of glycine, the simplest, have an asymmetric carbon atom (one that is attached to four different groups) and can therefore exist in distinct mirror-image forms, or enantiomers. One of the forms is "left-handed" or levorotatory (L-), while the other is "right-handed" or dextrorotatory (D-). Outside of the living world, wherever amino acids occur (with the interesting exception of organic matter in meteorites), they consist of equal amounts of the L- and D- forms in what is called a racemic mixture. However, terrestrial organisms use L-amino acids exclusively, giving rise to speculation about how this selection was originally made.
Amino acids and extraterrestrial life
Although only 20 different amino acids take part in the chemosynthesis of terrestrial proteins, over 100 naturally-occurring amino acids are known. This prompts the question as to whether extraterrestrial life could be built up from a different set of these fundamental organic compounds. To explore this possibility, Andrew Ellington and colleagues at the University of Texas, in 1998, grew a strain of the bacterium Escherichia coli which was incapable of manufacturing the essential amino acid tryptophan and therefore had to be supplied with it as a nutrient.1 The researchers also gave it the related synthetic amino acid fluorotryptophan. Although toxic to Earthly creatures, this might conceivably be an essential ingredient of life elsewhere. With 100% artificial substitute, the bacteria died within 3 cell divisions. With 95% fluorotryptophan and 5% normal tryptophan, however, the E. coli survived and slowly grew. After many generations, the bacteria started to divide at a greater rate as if mutations had arisen that were less susceptible to the synthetic chemical's toxic effects. Eventually, the bacteria were able to cope with a diet of 100% artificial substitute-growing extremely slowly but nevertheless surviving on this "alien" organic.
Two interesting possibilities are brought a step closer by this work. The first is that life on other worlds might have evolved and adapted to utilize a non-terrestrial mix of amino acids. If so, then any indigenous food-stuffs would have to be carefully analyzed before they were passed fit for human consumption. Extraterrestrial fruits, for example, containing amino acids of a different type or handedness to those found in our own bodies would either be indigestible or poisonous, a fact often overlooked in the Star Trek universe, where crew-members habitually sample the culinary delights of alien worlds without first checking their biomolecular credentials. The second possibility arising from the Texas study is that microbes from Earth might be encouraged to grow elsewhere in the Solar System (for example, on Mars), feeding on what would normally be toxic chemicals in the soil and releasing gases to help in terraforming the environment.
Reference
1. Cohen, Philip. "An Alien Diet," New Scientist, 14 (Aug. 1, 1998).


