nitrogen

Figure 1. Liquid nitrogen.
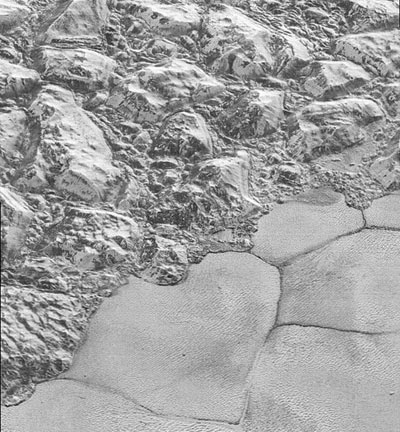
Figure 2. Solid nitrogen forming an almost flat plain, called Sputnik Planitia, on Pluto next to rugged terrain made of water ice.

Figure 3. Nitrogen cycle.
Nitrogen is a colorless, odorless gaseous element found in group VA of the periodic table. It was discovered by Daniel Rutherford in Edinburgh in 1772. Combined nitrogen occurs mainly as nitrates. Indeed, the name comes from the Greek nitron genes, which means "nitre forming"; nitre is potassium nitrate, commonly known as saltpeter.
Nitrogen is the most abundant gas in Earth's atmosphere (78 percent by volume) and is obtained from it by fractional distillation of liquid air. The most common isotope is 14N (99.76%). Nitrogen is also the sixth most common element in the universe. It is formed during hydrogen-burning in main sequence stars and red giants, via the carbon-nitrogen-oxygen cycle.
| atomic number | 7 |
| relative atomic mass | 14.00674 |
| electron configuration | 1s22s22p3 |
| atomic radius | 71 pm |
| oxidation states | -3, 5 |
| relative density | 1.25 |
| melting point | -209.9°C (-345.8°F) |
| boiling point | -195.8°C (-320.4°F) |
Chemistry of nitrogen
Nitrogen is almost insoluble in water. It does not burn, and if a burning substance is plunged into nitrogen the flames are quickly extinguished. Exceptionally, burning magnesium ribbon continues to burn feebly and magnesium nitride is formed.
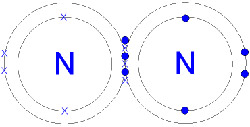 |
| Nitrogen molecule. Each atom three electrons with the other, forming a triple bond.
|
Molecular nitrogen is inert because of the strong triple bond between the two atoms, but it will react with some elements, especially the alkaline earth metals to give nitrides; with oxygen (e.g., the electric-arc process); and with hydrogen (see Haber process; it also forms N2 ligand complexes with group VIII transition metals. Activated nitrogen, formed in an electric discharge, consists of nitrogen atoms and is much more reactive.
Although few nitrogen compounds can be formed by direct action between nitrogen and other elements, there are many nitrogen-containing compounds, including a large number of organic compounds, e.g., proteins and nucleic acids, which are essential to life.
Although nitrogen can be obtained from the air after oxygen and carbon dioxide have been removed, this residue also contains traces of the inert gases as impurity. A purer product may be made by gently warming a strong solution of ammonium chloride (NH4Cl) and sodium nitrite (NaNO2):
NH4Cl + NaNO2 → NaCl + 2H2O + N2
In a double decomposition reaction sodium chloride (NaCl) and ammonium nitrite are formed, but the latter compound splits up almost as soon as it is formed to yield nitrogen and water.
Nitrogen fixation
Nitrogen fixation is the conversion of nitrogen gas into nitrogen compounds. Nitrogen usually reacts only at high temperatures and pressures. Industrially, it is fixed in the Haber process, the cyanamide process, and the electric-arc process.
Some bacteria (Rhizobium) in the root nodules of leguminous plants, and also some of those living free in the soil (e.g., Azotobacter), can fix nitrogen from the air as ammonia, nitrates, and nitrites. Some fungi and cyanobacteria also fix nitrogen. See also nitrogen cycle.
Cyanamide process
In the first stage of the cyanamide process, calcium carbide (CaC2) is made by heating calcium carbonate with coke. Nitrogen is then passed through the finely-divided calcium carbide at 1000°C, giving calcium cyanamide (CaCN2). The calcium cyanamide is then decomposed by steam to give calcium carbonate (for recycling) and ammonia.
Electric-arc process
The electric-arc process is a process for nitrogen fixation, now largely uneconomical. Air is blown through an electric arc at 1000°C, and nitric oxide is formed. It is converted into nitric acid as in the Ostwald process.
Haber process
See main article.
In the Haber process, ammonia is produced from hydrogen and atmospheric nitrogen.
Compounds of nitrogen
Nitrogen forms mainly trivalent and pentavalent compounds.
Nitrates
A nitrate is a salt of nitric acid. Nitrate salts contain the nitrate ion (NO3-). Some nitrates, notably saltpetre (potassium nitrate, KNO3) and Chile saltpetre (sodium nitrate, NaNO3) are important naturally occurring chemicals; others are made by the action of nitric acid on the metal or its salts. Nitrates are used in medicine, as food preservers, fertilizers, and explosives, and as a source of nitric acid. Esters of nitric acid (RONO2) are also called nitrates.
Nitrites
Nitrites are salts (or esters) of nitrous acid (HNO2) and are mild reducing agents.
nitric oxide
Nitric oxide (NO) is a colorless gas formed in the electric-arc process; it is readily oxidized further to nitrogen dioxide. The NO molecule is unusual in having an odd number of electrons, and so gives the nitrosyl ions NO+ and NO–. Melting point –164°C, boiling point –152°C.
Nitrous oxide
Nitrous oxide (N2O), also known as nitrogen monoxide or laughing gas, is a colorless gas with a sweet smell that is sometimes used as an anesthetic or analgesic during surgical or dental operations, and an aerosol propellant. Nitrous oxide is soluble in water, ethanol, and sulfuric acid. On heating above 520°C, it is decomposed to nitrogen and oxygen and will thus support the combustion of many compounds. It also reacts with oxygen to form nitrogen dioxide. However, it is relatively unreactive, being inert to halogens and alkali metals. Melting point –91°C, boiling point –88°C.
Nitrous oxide can be prepared by cautiously heating a mixture of ammonium sulfate and sodium nitrate. By a double decomposition reaction, sodium sulfate and ammonium nitrate are formed. The latter compound then decomposes to yield nitrous oxide and water. The gas is often collected over warm water as it is soluble in cold. It can also be prepared by reducing nitric acid, or by reacting sodium nitrite, sulfuric acid and iron (II) (ferrous) sulphate.
| density | 1.97 g dm-3 |
| melting point | -90.8 °C |
| boiling point | -88.5 °C |
Nitrogen dioxide
Nitrogen dioxide (NO2), a pungent-smelling, red-brown toxic gas in equilibrium with its dimer (N2O4), is a constituent of automobile exhaust and smog. It is made by the action of concentrated nitric acid on copper, and dissolves in water to give a mixture of nitrous and nitric acids. It is also formed by the reaction of oxygen with nitrogen monoxide. A powerful oxidizing agent, it is used in the manufacture of sulfuric acid and in rocket fuels. It is also an intermediate in the manufacture of nitric acid. Its presence in the atmosphere contributes to the formation of acid rain and the depletion of the ozone layer. Melting point –11°C, boiling point 21°C.
Nitrogen tetroxide
Nitrogen tetroxide (N2O4) is a yellow-brown liquid that is among the most common storable oxidizers used by liquid-propellant rocket engines today. Like nitric acid, it is hypergolic (reacts upon contact with) hydrazine, MMH (monomethyl hydrazine), and UDMH (unsymmetrical dimethyl hydrazine). It is used, for example, with MMH in the Space Shuttle orbital maneuvering system. Although it can be stored indefinitely in sealed containers, its liquid temperature range is narrow and it is easily frozen or vaporized.
Nitrogen cycle
The nitrogen cycle is the cycle of chemical changes that involves the exchange of nitrogen between the air and the soil.
Free nitrogen (N2) in the atmosphere cannot be absorbed directly by plants or animals. Nitrogen fixation – industrial (producing fertilizers) or by microorganisms – yields combined nitrogen as ammonia or nitrates, which can be absorbed from the soil by plants, which use them to make protein. Herbivores ingest nitrogen by eating these plants. Excretion products and animal and plant remains return nitrogen to the soil as complex compounds which are converted by fungi and bacteria to ammonium salts, which may then be oxidized to nitrites and nitrates by other bacteria. These are either reused by plants, or converted to nitrogen (by denitrifying bacteria) which returns to the air, thus completing the cycle (Fig 3).
Nitrogen and life
Nitrogen, along with hydrogen, carbon, and oxygen, is essential for life as we know it. Nitrogen is of central importance in the formation of amino acids, which are themselves essential components of proteins and nucleic acids. As an element, nitrogen is not particularly reactive because of its great affinity for itself. Nitrogen molecules are composed of two nitrogen atoms, linked by a triple bond that is difficult to break. However, the nitrogen in the atmosphere can be converted without the expenditure of too much energy into ammonia, which is the source of nitrogen in biological compounds.
Animals and plants can't use free nitrogen gas. Bacteria living in the roots of plants such as peas, take in atmospheric nitrogen, and, along with other microorganisms, convert the gas to ammonium salts and to nitrates. Plants get the nitrogen they need from the inorganic nitrogen compounds in the soil. Animals obtain the nitrogen they need from plants or from other animals.
The nitrogen content of soil, where the nitrogen cycle starts, is enriched and renewed by excretion and decay of animals and plants. Some of the trapped nitrogen is returned to the air as bacteria in soil decompose the nitrogen compounds and release the element back into its gaseous form.
Humans breathe nitrogen in and out of their lungs all the time, without any serious side effects. The nitrogen gas dissolves slightly in the blood and circulates around the body harmlessly. Under pressure however, such as when a person dives into deep water, the amount dissolved nitrogen increases. If the decompression is slow and careful, the dissolved nitrogen comes out of the body fluids and can be removed through the lungs, but, if decompression is too rapid, the 'bends' causes great pain and even death. This decompression sickness is caused by bubbles of nitrogen rapidly coming out of solution in the bloodstream.


