ethanol
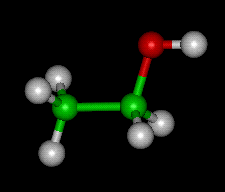
Ball-and-stick model of an ethanol molecule; carbon atoms = green, hydrogen = gray, oxygen = red.
Ethanol (C2H5OH), also known as ethyl alcohol, is a colorless alcohol that is soluble in water. It is the active principle in intoxicating (alcoholic) drinks, such as beer and wine, in which it is produced by the fermentation of sugar using yeast:
C6H12O6 → 2C2H5OH + 2CO2
Ethanol is miscible with water with the evolution of heat and a contraction in volume; pure ethanol absorbs water vapor. Many gases are more soluble in it than they are in water. Some inorganic salts and many organic compounds are soluble in ethanol.
Ethanol is oxidized to ethanal (a.k.a. acetaldehyde) or ethanoic acid; with nitric acid a variety of products, including glycolic acid and oxalic acid are formed. Ethanoates are formed by the action of sodium, calcium, aluminum, and some other metals on ethanol. It reacts with acids to give esters. With sulfuric acid it yields ether, ethene, or ethyl hydrogen sulfate. Bleaching powder converts it to chloroform, while chlorine gives chloral.
The main industrial use of ethanol is as a solvent although at one time it was a major starting point for making other chemicals. For this it was produced by the fermentation of molasses. Now ethene has replaced ethanol as a raw material and industrial ethanol is made by the hydrolysis of ethene.
| density relative to water | 0.789 at 0°C |
| melting point | -114.3°C (158.8 K) |
| boiling point | 78.4°C (351.6 K) |
| molar mass | 46.06844 g/mol |
Types of ethanol
Methylated spirit is an industrial form of ethanol. It contains 5% methanol, which is extremely poisonous, and enough pyridine to give it a foul taste. It is dyed purple and used as a solvent and fuel.
Rectified spirit is a constant-boiling mixture of ethanol and water. It is made by distilling ethanol-water mixtures and contains 95.6% ethanol.
Pure ethanol, also called absolute alcohol, is made from distilled ethanol (a constant-boiling mixture containing 95.6% ethanol) by the addition of sodium metal and a drying agent.
What happens to ethanol inside the body?
Ethanol bypasses the normal processes of digestion in the small intestine and stomach. Instead of being broken down, it is absorbed intact through the stomach wall (20%) and small intestine (80%), passes into the bloodstream, and is then absorbed by many of the body's tissues.
Alcohol is diluted by water in the body. Since there is more water in muscle than in fat, a muscular person who consumes alcohol will end up with a lower concentration of alcohol in their system, and consequently be less intoxicated, than a person of the same weight and gender with a higher percentage of body fat.
Women typically have a higher proportion of fat to muscle than men. Consequently, women tend to have a higher systemic concentration of alcohol and suffer more severe intoxication effects than men of equal weight who have consumed the same quantity of alcohol. If the alcohol concentration is high enough (due to rapid consumption or high percentage of ethanol), the pyloric sphincter between the stomach and small intestine may spasm, enabling a higher percentage of alcohol to enter the blood via the stomach. Since the alcohol cannot leave the stomach to be absorbed into the blood more quickly in the small intestine, fast drinking can actually postpone intoxication.
A major portion of systemic ethanol is metabolized within the liver. Any alcohol that is not thus metabolized travels to other organs and to the nerves depressing the central nervous system. Alcohol is one of a few compounds that can cross the blood-brain barrier, which is what causes intoxication. Acetaldehyde, a by-product of alcohol breakdown, is one of the most common neurotoxins found in people today, resulting in brain damage in tens of millions of people.


